INTRODUCTION
COVID-19 was originated in Wuhan, China, at the beginning of December 2019. Since then a rapid spread has beaten the entire world (1). Mexico has been one of the most affected countries ranking twelfth in number of new cases and fourth in mortality as of January 20th, 2021 (2). High infectivity and considerable variability had defined the clinical presentation of the disease. More than 40 % of patients are symptomless, 30 % to 45 % manifest mild symptoms, and 15 % manifest severe symptoms with hospitalization or intensive care (3). COVID-19 severity has been linked to elevated oxidative stress, exaggerated immune response, uncontrollable liberation of proinflammatory cytokines, and activation of coagulating factors, all of which contribute to severe inflammation (4,5). The effect of vitamin D on maintaining bone health and calcium-phosphorus metabolism is well known. But also, it possesses anti-inflammatory antifibrotic and antioxidant qualities. It modulates the immune system (6,7) and its anti-inflammatory function explains the beneficial effect on immune responses and antimicrobial agent production (8). Also, vitamin D regulates the renin-angiotensin system and expression of angiotensin-converting enzyme 2 linked to a lung protective effect (9,10).
Scientists have centralized their attention on factors that can control or prevent COVID-19 severity. Vitamin D has been suggested for such intentions (11), but its role on SARS-CoV-2 severity is still under investigation (12,13). D'avolio et al. described lower vitamin D levels in patients with COVID-19 symptomatology for the first time (14). There are observational studies that have determined an association between vitamin D levels and COVID-19 severity, but their findings are not consistent (11,15-16); and the literature is limited in Hispanic American populations (17). The objective of the present study was to compare vitamin D concentrations and prevalence of vitamin D deficiency between individuals with no COVID-19 and patients with mild and severe COVID-19. Also, to analyze the association between vitamin D deficiency and COVID-19 severity in Northeast Mexico.
MATERIALS AND METHODS
This was a cross-sectional study. A non-random sampling was carried out in two sites, a referral diagnostic primary care center and an intensive care unit from a secondary care hospital. All individuals with suspected symptoms of COVID-19 who attended the referral center for a real-time polymerase chain reaction (PCR) test between March and September 2020 were consecutively included (n = 181). Concurrently, every patient admitted to intensive care with a diagnosis of COVID-19 was also consecutively included (n = 116). Inclusion criteria consisted of age ≥ 18 years and no pregnancy. The sample size in each group was sufficient for a power greater than 80 % and alpha greater than 95 % given the 10 % observed outcome in the unexposed group and the unadjusted odds ratio obtained. The protocol was approved by the "Dr. Bernardo Sepulveda" Hospital's Committee of Ethics and Health Research (HMBSSSNL-2020/878). An informed consent was provided by all the participants.
COVID-19 SEVERITY STATUS
COVID-19 severity status was classified as negative (individuals with suspected symptoms of COVID-19 with a negative PCR test at the referral diagnostic primary care center), mild (individuals with suspected symptoms of COVID-19 with a positive PCR test at the referral diagnostic primary care center and who were maintained on ambulatory care), and severe (patients admitted to intensive care with a COVID-19 diagnosis).
VITAMIN D STATUS
Serum 25(OH)D was estimated using the 25-OH Vitamin D kit (ARCHITECT i; Abbott Laboratories; reference 5P02-25) and the Vitamin D controls (ARCHITECT; Abbott Laboratories; reference 5P02-10) on Architect i2000 SR analyzer 5P02 (Abbott Diagnostics, Chicago, USA). Values > 40 ng/mL were considered above normal, values between 30 and 39 ng/mL normal, values between 20 and 29 ng/mL mild vitamin D deficiency, values between 10 and 19 ng/mL moderate vitamin D deficiency, and values < 10 ng/mL severe vitamin D deficiency. In addition, a cut-off value of 25(OH)D < 20 ng/mL was used for combining moderate and severe vitamin D deficiency.
OTHER BIOCHEMICAL MEASUREMENTS AND DEMOGRAPHIC DATA
Lactate dehydrogenase (LDH) (U/L), C-reactive protein (mg/L), D-dimer (ng/ml), and fibrinogen (mg/L) were obtained. Also, leukocytes (× 109/L), lymphocytes (× 109/L), neutrophils (× 109/L), platelets (× 109/L), and prothrombin time (seconds). Blood samples were taken at admission to intensive care (severe COVID-19 patients) or alongside with the PCR test at the referral diagnostic primary care center (mild and no COVID-19 individuals). All laboratory measurements were prospectively collected from the clinical chart, as well as sex, age, and body mass index (BMI).
STATISTICAL ANALYSIS
Frequencies were obtained for the categorical variables, as were means and standard deviations for the non-categorical variables with normal distribution; otherwise, medians and interquartile ranges (percentile 25th and 75th) were calculated. Vitamin D deficiency point prevalence and 95 % confidence intervals (CI) were estimated for each COVID-19 severity group. The chi-square and non-parametric one-way ANOVA tests with post-hoc analysis were used to make comparisons between groups. Non-parametric coefficient correlations were also estimated between vitamin D concentration and inflammatory biomarkers. The association between vitamin D and COVID-19 severity was analyzed with a multivariate logistic ordinal regression using COVID-19 status as the dependent variable (no, mild and severe), vitamin D as the independent variable (normal/above normal, mild, moderate, and severe deficiency), and sex, age, BMI, LDH, C-reactive protein, D-dimer, and fibrinogen as control variables.
RESULTS
The study population's mean age was 46.0 ± 17.4 years and 51.1 % were male. Male sex, mean age, mean BMI, and median blood measurements were higher in severe COVID-19 patients than in individuals with no COVID-19 (p ≤ 0.05). All blood test results were less favorable in the severe COVID-19 group when compared to the non-COVID-19 group. As well as most of the blood tests in the mild group when compared to the non-COVID-19 group (Table I).
Table I. Characteristics of the study population by COVID-19 severity status
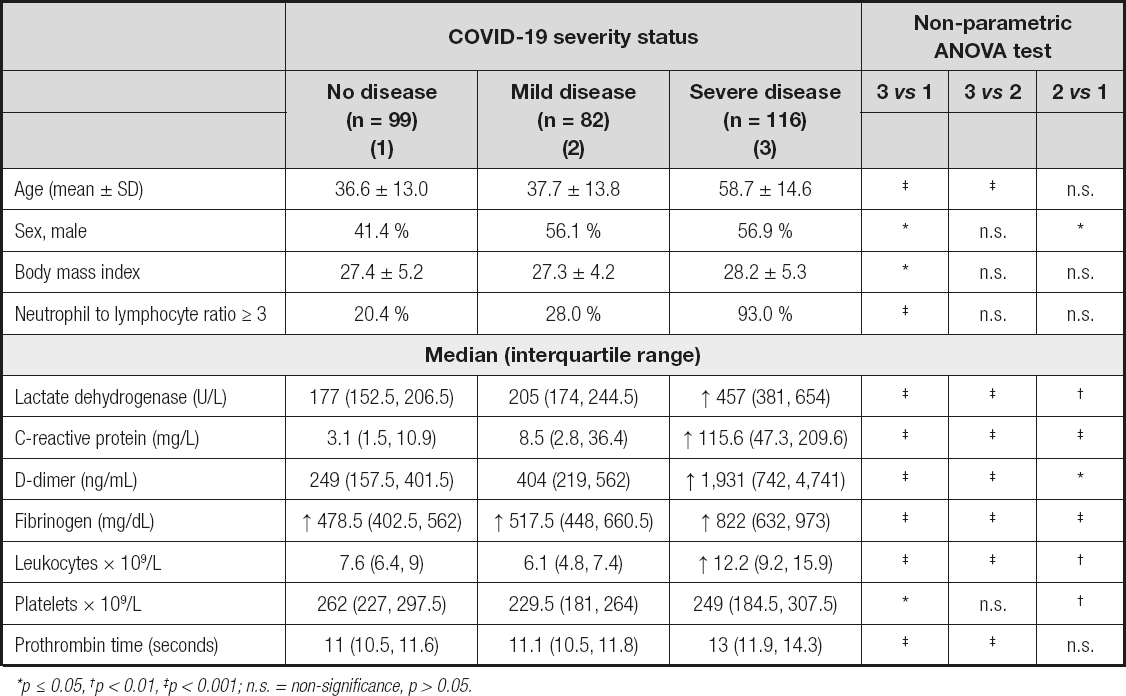
*p ≤ 0.05, †p < 0.01, ‡p < 0.001; n.s. = non-signifi cance, p > 0.05.
VITAMIN D AND COVID-19 SEVERITY STATUS
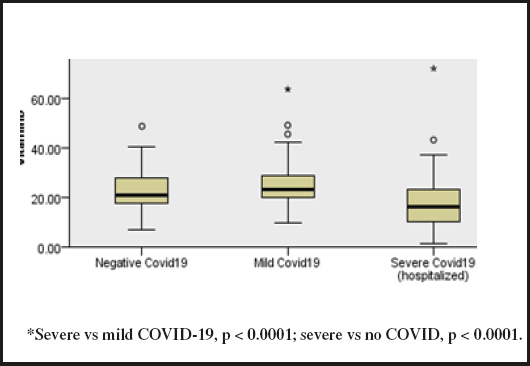
Median vitamin D was lower in the severe COVID-19 group (16.3 ng/mL; IQR, 10.2, 23.2) than in the mild (23.4 ng/mL; IQR, 19.9, 29.0) or no COVID-19 group (21.0 ng/mL; IQR, 17.6, 27.9) (p < 0.0001) (Fig. 1). There were no vitamin D differences by sex, but it was negatively correlated with age (rho = - 0.13, p < 0.02). Vitamin D was also negatively correlated with LDH (rho -0.20, p < 0.0001) and D-dimer (rho -0.27, p < 0.0001).
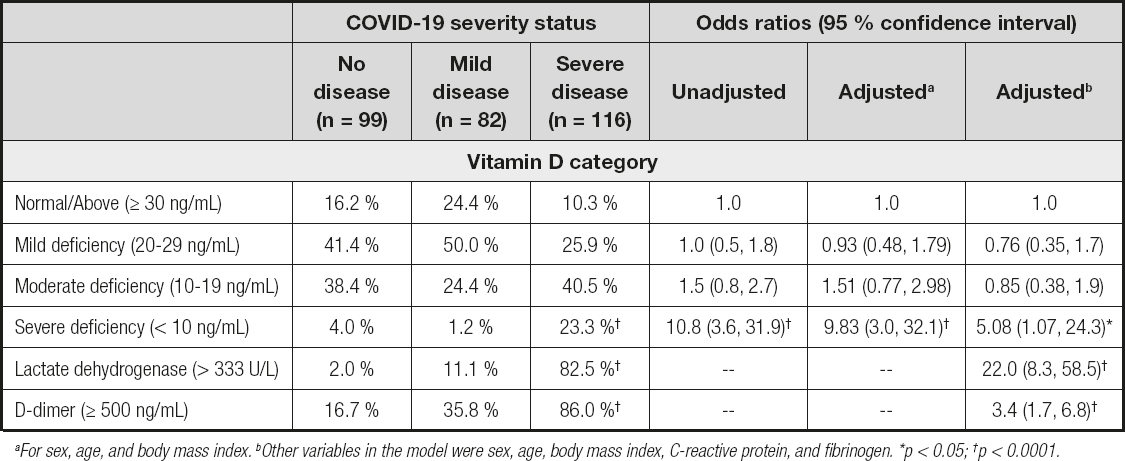
The prevalence of vitamin D < 20 ng/mL was 63.8 % (95 % CI, 54.7, 72.0) in the severe COVID-19 group, 25.6 % (95 % CI, 17.4, 36.0) in the mild COVID-19 group, and 42.4 % (95 % CI, 33.2, 52.3) in the non-COVID-19 group. Severe vitamin D deficiency increased the odds of severe COVID-19 by five times regardless of sociodemographic, BMI, and inflammatory blood markers (Table II).
DISCUSSION
The objective of the present study was to compare vitamin D concentrations and prevalence of severe vitamin D deficiency between individuals with no COVID-19 and patients with mild and severe COVID-19. There was a difference in prevalence of vitamin D deficiency between groups (< 20 ng/ml), although it was not in ascending order from non-COVID-19 to moderate and severe COVID-19. De Smet et al. (18) reported vitamin D deficiency rates increasing from 55 % in stage 1 (pulmonary ground-glass opacities) to 67 % in stage 2 and 74 % in stage 3 (diffuse alveolar damage and fibrosis). AlSafar et al. (19) did not find any differences by COVID-19 severity. They identified 76 % of vitamin D deficiency in asymptomatic, 65 % in mild, 67 % in moderate, and 61 % in severe COVID-19 patients (p > 0.05). Comparison of vitamin D deficiency in COVID-19 patients in intensive care between countries shows 61 % in the United Arab Emirates (19), 72.1 % in Italy (20), 74 % in Belgium (18), 96.8 % in India (11), and 63.8 % in Northeast Mexico (this study). The prevalence in healthy populations varies. It has been estimated at 37.3 % in European countries (21) and at 40 % (summer time) and 65 % (winter time) in Northeast Mexico (22). Vitamin D deficiency was not associated with sex but with age - the older the age, the lower the vitamin D concentration, contrary to De Smet et al. (18), who identified that male sex doubled the odds of vitamin D < 20 ng/mL but no association could be found with age. Adami et al. (20) found no association with age or sex. Major causes of vitamin D deficiency are inadequate exposure to sunlight, obesity, and kidney disease, among others (23,24). Variations in the causes of deficiency are probably also the origin of differences in prevalence values in addition to severity status.
We identified that the relationship between vitamin D, D-dimer, and LDH was inverse, indicating that the intensity of the inflammatory response was high with low levels of vitamin D. Hernandez et al. (17) also identified an inverse correlation with D-dimer levels. Adami et al. (20) did not find such association but did find one with C-reactive protein. LDH is an important marker of lung damage and D-dimer reflects the progression of disease toward an unfavorable clinical outcome (25). In this study, LDH, D-dimer, C-reactive protein, fibrinogen, and leukocyte values were significantly higher in severe COVID-19. Besides, D-dimer increased 3 times and LDH increased more than twenty times the odds of severe COVID-19. Vitamin D itself is linked to anti-inflammatory antifibrotic qualities, so if vitamin D is low, anti-inflammatory antifibrotic properties fail, and prevention or reduction of COVID-19 severity also fails.
Median vitamin D concentrations were lower in severe COVID-19 than in mild COVID-19 patients with a difference of -7.4 ng/dL (95 % CI, -10.5, -4.3), which was consistent with a review study showing a weighted mean difference of −7.2 ng/mL (95 % CI, -10.9, -4.3) between severe and less severe COVID-19 patients (26). De Smet et al. (18) also reported lower median vitamin D in most severe versus less severe COVID-19 patients. Vitamin D deficiency (< 12 ng/mL) increased 5 times the odds of severe COVID-19 regardless of sex, age, BMI, LDH, D-dimer, C-reactive protein, and fibrinogen. Ye et al. (15) reported an odds ratio of 15.2 (95 % CI, 1.2-187.5) regardless of age, sex, renal failure, diabetes, and hypertension in China. Also, Jain et al. (11) found an association between vitamin D deficiency (< 20 ng/dL) and intensive care admission due to severe COVID disease in India. However, the literature is not consistent. Studies by Cereda et al. (27), Hernández et al. (17), and Szeto et al. (28) have not found an association. AlSafar et al. (19) did not identify that vitamin D < 20 ng/mL increased the odds of severe COVID-19, but they did with vitamin D < 12 ng/mL, independently of sex, age, and comorbidities. The review study by Kazemi et al. (26) estimated an overall effect size of 2.6 (95 % CI: 1.7, 4.0) on a composite measure of COVID-19 severity (at least 1 of the following outcomes: acute respiratory distress syndrome, mechanical ventilation, ICU admission, length of hospitalization, and death). But no significant association was found when intensive care was the only measure of severity (16,17,29).
LIMITATIONS
No data was available on prior vitamin D supplementation. So, prevalence estimation on vitamin D deficiency might not be accurate. However, the association result is not expected to be affected, since such an association occurred between current vitamin level and current disease severity, regardless of how that level was reached. Given the cross-sectional study design, there is no certainty in directionality - if vitamin D deficiency influenced disease severity, or if deficiency was a consequence of disease severity. A cohort design is needed for distinguishing directionality. Better yet, clinical trials on vitamin D supplementation are on their way. Chronic conditions such as hypertension, diabetes, cancer, or cardiovascular diseases may contribute to poor COVID-19 prognosis; we did not have this information available, but we had inflammation biomarkers instead. Finally, only non-pregnant adults were recruited from one referral diagnostic primary care center and one intensive care unit. So, caution is needed when generalizing results to children and pregnant women. Further research is needed with multicenter participation considering populations younger than 18 years and pregnant women.
CONCLUSIONS
This study is the first report of vitamin D deficiency in no-, mild, and severe COVID-19 subjects in Northeast Mexico. Lower vitamin D concentrations and a higher prevalence of vitamin D deficiency were present in patients with severe COVID-19. We found an inverse relationship between vitamin D, LDH, and D-dimer, and an association between vitamin D deficiency and COVID-19 severity. Yet, large-scale observational studies and randomized controlled trials of vitamin D supplementation for controlling COVID-19 severity are necessary in Hispanic American populations.