INTRODUCTION
Worldwide, obesity is rapidly becoming a global health hazard, which causes healthcare systems a substantial financial burden. According to the World Health Organization, the prevalence of obesity has tripled since the mid-1970s. Globally, more than 1 billion adults are overweight, and 650 million adults and 124 million children and adolescents have obesity. Moreover, the societal ramifications of this grim development are wide ranging - approximately, 90 million U.S. adults (42.4 %) now live with obesity, which is disproportionately present in socioeconomically disadvantaged persons and affects 57 % of black women (1). Obesity is also a major risk factor for, and is tightly associated with, many adverse health conditions, including cardiovascular diseases, type-2 diabetes, metabolic disorders, and multiple types of cancers. Under this disease burden, the available methods of obesity treatment have greatly lagged behind that of any other chronic metabolic diseases in primary care settings.
It is well known that insufficient energy consumption or excessive energy intake by the human body can result in adipose tissue accumulation, which is a major pathological feature of obesity. The increase in fat will generate an accumulation of fatty acids and triglycerides, which mainly exist in white adipose tissue (WAT). In obesity, an increase of WAT can also cause a chronic low-grade pro-inflammatory scenario, due to a dysregulation of secreted molecules. Meanwhile, there is another adipose tissue depot, which serves as a key site for heat production in mammals and has been previously targeted to promote weight loss: the brown adipose tissue (BAT). Recently, increasing lines of evidence have suggested that BAT uses fatty acids to generate heat instead of ATP to protect against obesity through the process of non-shivering thermogenesis (NST) (2). Increasing the metabolic activity of this tissue plays a crucial role in improving glucose and lipid homeostasis, increasing energy expenditure, and reducing weight.
A phenomenon called white adipose tissue browning is seen in various animal models, in which, in response to cold exposure, β-adrenergic and exercise stimuli, WAT cells are transformed into brown adipocyte-like cells. In recent years, a number of studies using animal models have acknowledged that BAT transplantation has beneficial effects on obesity and associated disorders including elevation of lipid profiles, reduction of tissue inflammation, improvement of energy expenditure, and reversal of even weight gain. Meanwhile, emerging experimental studies have demonstrated that changes in the fundamental morphologic, physiologic, or other metabolic activities of adipose tissue, such as the browning of adipose tissue, can affect outcomes in obesity, which unequivocally confirmed that WAT browning could be a feasible therapeutic target of obesity in rodents (3,4). Even so, the underlying mechanisms have not been extensively reviewed to date. In the present study, as a systematic review, the relationship between WAT browning and obesity has been studied, and regarding the evidence achieved from studies, new therapeutic approaches in this field have been proposed.
TYPES OF ADIPOSE TISSUE AND THEIR FUNCTION
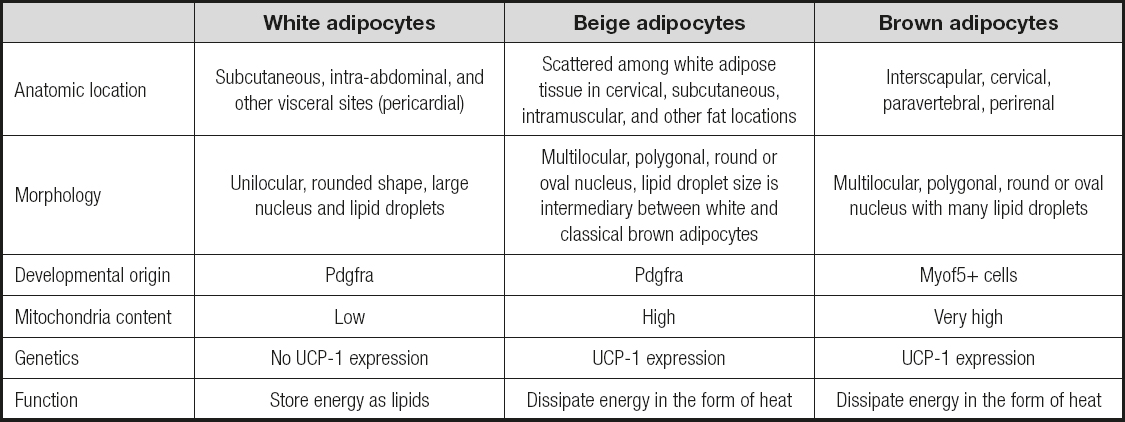
As one of the largest endocrine organs in the body, adipose tissue is involved in a large number of physiological processes. Generally, adipose tissue serves as the central player to store excess energy in the form of fat, yet it differs in its ability for energy expenditure and storage based on its anatomical location, mitochondrial density, and thermogenic potential. Traditionally, there are two main types of adipose tissue that differ in their origin, morphology, and function in the body: WAT and BAT. WAT is responsible for energy storage in the form of triglycerides, while the known function of BAT is specialized to maintain body temperature via heat production. The characteristics of the different kinds of adipose tissue are shown in table I.
WHITE ADIPOSE TISSUE
WAT is the predominant deposit of adipose tissue in human adults, representing 10-20 percent of body weight in healthy subjects. It is composed of white adipocytes, which is a complex endocrine organ exhibiting a large nucleus and unilocular lipid droplets located in the periphery. In humans, white adipocytes are derived from mesenchymal stem cells (MESCs), common precursors for chondrocytes, osteoblasts, and adipocytes. MESCs initially differentiate into pre-adipocytes, cells that are morphologically indistinct from MESC precursors but committed to WAT or BAT lineages. Sequentially, a signalling cascade of events occurs to develop these cells into mature adipocytes terminally. The process of adipocyte terminal differentiation and the molecular regulation mechanisms which maintain the adipocyte in this differentiated state are well characterized, and several known transcription factors, including peroxisome proliferator-activated factor gamma (PPARγ) and CCAAT/enhancer binding-proteins C/EBPβ, predominantly regulate this differentiation.
The main function of WAT is to store energy in the form of triacylglycerols (TAG), which can be mobilized via lipolysis from fat stores to meet energy demands such as fasting and physical exercise. The process of lipolysis is tightly regulated by complicated regulatory mechanisms involving lipolytic hormones such as catecholamines, which activate cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) and result in phosphorylation of hormone-sensitive lipase (HSL) and perilipin 1 (Plin1). WAT also has important endocrine functions, being able to secrete adipokines and cytokines such as adiponectin, interleukin-6 (IL-6), leptin, and tumor-necrosis factor alpha (TNFα), which are involved in the regulation of diverse metabolic processes. However, WAT may fail to regulate energy homeostasis when it is dysfunctional during sustained excess energy intake. Excessive fat accumulation has also become the major risk factor for the development of a range of metabolic disorders including atherosclerosis, hypertension, type-2 diabetes, and cancer, thus adipokines are potential targets for these chronic diseases.
BROWN ADIPOSE TISSUE
BAT is a thermogenic organ containing high mitochondrial density and multilocular lipid droplets, thus enables homoiothermic mammals to defend themselves from the cold environment and to maintain body temperature through non-shivering thermogenesis. BAT is metabolically active in individuals with high energy requirement such as newborns, small animals with high-rate metabolism, people under cold exposure, or hibernating animals. It has been estimated that BAT is primarily distributed throughout the interscapular region, accounting for 1-5 % of body weight. Recent studies have provided compelling evidence that BAT cells are not only different from WAT cells histologically, but that they also stem from different precursor cells and display different molecular signatures (5). Several genes that act as transcription co-regulators in the development of BAT adipocytes have been identified, such as the PR domain containing 16 (PRDM16), cytochrome C, type-II iodothyronine deiodinase (DIO2), β3 adrenergic receptor (β3-AR), and the peroxisome proliferation-activation receptor and coactivator 1α (PGC-1α).
Nowadays, it is well established that BAT is strongly governed by the sympathetic nervous system (SNS), which influences BAT activity and metabolism through a variety of stimuli, including cold exposure and exercise. Cold exposure is the most well-studied means to activate BAT, as non-shivering thermogenesis is highly associated with BAT in mammals. Upon stimulation, norepinephrine binding to β3 adrenergic receptors will be released to activate a cascade of metabolic events in the membrane of brown adipocytes, leading to increases in fatty acid β-oxidation and ultimately heat production (6). Data have illustrated that BAT thermogenesis is inhibited in the absence of all three β-adrenergic receptors leading to a rapid drop in core body temperature, which supports the critical role of β3-AR in thermogenesis (7). Mechanistically, the release of β1-adrenergic receptors enhances proliferation of brown preadipocytes, whereas β3-AR mainly acts to regulate the differentiation of mature brown adipocytes through cAMP-dependent signaling pathways (8). Deficits in either of them alter BAT functions. Apart from the well-known cold-activated pathways, the control of BAT thermogenesis has been closely linked to a number of hypothalamic nuclei to allow diet-induced thermogenesis, such as the energy homeostasis regulatory pathway.
In summary, the enormous effect on energy utilization makes this special type of adipose tissue an appealing target for new therapeutic approaches in order to tackle obesity and other metabolic disorders.
WHITE ADIPOSE TISSUE BROWNING
ADIPOCYTE PRECURSORS IN ADULT ANIMALS
Previously, it was thought that brown and white adipocytes were two separate differentiating lineages that diverged developmentally from the same adipocyte progenitor cells (APCs, also known as adipose stem cells, ASCs). However, recent evidence confirms that classical brown adipocytes develop along the myogenic lineage and arise from precursors expressing myogenic factor 5 (Myof5+), whereas white adipocytes derive from precursors positive for platelet-derived growth factor receptor alpha (Pdgfra+). Myof5 initiates the program of myogenic differentiation by encoding a basic-helix-loop-helix transcription factor, whereas Pdgfra determines progenitor commitment to beige adipogenesis via the encoding of a tyrosine kinase (9). Therefore, brown adipocytes may differentiate into skeletal muscle cells, and both types are functionally interrelated with each other subsequently as well. Beige adipocytes derive from the same precursors as white adipocytes, providing evidence that beige adipocytes arise from a completely de novo development as compared to BAT.
Beige adipocytes are found interspersed within WAT with a multilocular morphology similar to that of brown adipocytes. Upon stimulation, beige adipocytes have an increased capacity for thermogenesis and fuel oxidation. Unlike brown adipocytes, inducible beige adipocytes are dependent on external stimuli to induce the expression of UCP1, which is a distinctive feature of beige adipocytes. In the basal state, only a very low level of thermogenic gene expression, similar to that of white adipocytes, will be released by beige adipocytes; however, high levels of UCP1 that resemble brown adipocytes will be expressed by beige adipocytes if fully stimulated. Therefore, the beige adipocyte's capacity to switch between energy dissipation and energy storage usually depends on the type of stimuli it receives, an ability that classic brown adipocytes lack.
In rodents, induction of beige adipose tissue activity is associated with factors produced by several types of tissues and cells through autocrine, paracrine, and systemic mechanisms. M2 (alternatively activated) macrophages produce catecholamines to induce a gene expression profile enriched for proliferation, adhesion, migration, lipolysis, foam cell differentiation, extracellular proteolysis and matrix remodeling, and foam cell differentiation, thereby increasing energy expenditure in mice. Moreover, factors such as fibroblast growth factor-21 (FGF21), BMP7, BMP8b, natriuretic peptides, β-aminoisobutyric acid (BAIBA), and prostaglandins can influence the differentiation of beige adipocytes. All of these factors, which are capable of increasing energy expenditure via various mechanisms, play a positive role on weight loss by improving insulin sensitivity and glucose homeostasis in animals fed a high-caloric diet.
Recently, studies have demonstrated that beige adipose tissue is a distinct type of adipose tissue that defends the body against obesity through thermogenesis, with some beige-selective marker detectable in the WAT of adult animals after exposure to adrenergic agonists or cold environment, such as transmembrane protein 26 (TMEM26), CD137, and other thermogenic markers, including transcriptional co-regulatory PRDM16 and PGC-1β, and mitochondrial genes Cox7a1 and Cox8b. In a previous study, TMEM26 expression was widely detected in all seven depots and was mainly expressed in the visceral adipose tissue (VAT) and the inguinal depots, which was similar to the expression pattern of UCP1. Early studies have shown that the expression level of TMEM26 was significantly increased after treatment with emodin, and more multilocular lipid droplets were found in WAT (10). Moreover, the study by Auffret et al. demonstrated that after the knockout of the prolactin receptor gene, white adipocytes of mice transformed into beige adipocytes. Body weight and adipose tissue mass were significantly reduced in obese mice, and mRNA expressions of PGC-1α, UCP1, and TMEM26 were also significantly up-regulated, suggesting that the activation of TMEM26 can promote white browning (11).
However, there has still been much debate about whether cold-induced beige adipocytes are derived from the de novo differentiation of adipogenic precursor cells or derive from mature white adipocytes directly. Several lines of evidence in recent years now suggest that direct conversion of white to beige adipocytes can be a dominant mechanism of the WAT browning process. Firstly, little changes in adipocyte number or DNA content have been found during the induction of beige adipocytes. Secondly, most beige adipocytes arise from non-dividing cells, which make mature adipocytes the most likely source of such cells. On the other hand, the process of transdifferentiation from white adipocytes to beige adipocytes can be reversible. This process has been supported by an elegant in vivo lineage-tracing study using permanent and transient fluorescent cell labelling in transgenic mice. To sum up, further studies are needed to know the stimuli that maintain and enhance WAT browning over time.
BROWNING AS A SYMPATHETIC EVENT
Adipose tissue has allowed mammals to adapt to changes caused by nutrient availability, environmental conditions, and energy demand. White adipose tissue browning is a multistage process, which mainly occurs by interacting with sympathetic stimulation and norepinephrine (NE), with the β3-AR involved triggering a cascade of signal transduction. The primary sympathetic activator of brown adipocytes is a reduction in temperature, which can induce a massive thermogenic response. This effect is mainly mediated by the activation of the sympathetic nervous system.
In general terms, a central regulator of adaptive thermogenesis mediated by the nervous system is hypothalamic AMP-activated protein kinase (AMPK). Activated by low intracellular energy stores, AMPK reduces ATP consumption and switches intracellular processes to ATP production. Inactivation of AMPK in the ventromedial hypothalamus (VMH) results in enhancement of sympathetic output to WAT and BAT, which conveys WAT browning and BAT thermogenesis. Deletion of AMPKα1 (the AMPK subunit) in the VMH inspires a thermogenic program in BAT, which represents a potential target for the treatment of obesity and other metabolic diseases (12). Even though it seems to bring some metabolic benefits by decreasing hypothalamic AMPK activity, a loss of AMPK expression in adipocytes could exacerbate hepatic steatosis and insulin resistance through impairing BAT function. In order to maximize metabolic benefits, potential therapies targeting AMPK activity must be tissue-specific.
Other modulators of thermogenic activity may also determine WAT browning. For example, the interaction of NE with β3-AR activates the glutamine synthetase (Gs) protein and, in turn, stimulates adenylyl cyclase (AC), which finally induces the production of PKA activator-cAMP. This PKA-dependent transduction signal will end with the overexpression of UCP1 and other thermogenic proteins. The activation of the mitogenic pathway by the p38 Map-kinase that is directly involved in PGC-1α and UCP1 overexpression also plays an essential role in the regulation of thermogenesis. Subsequently, this pathway stimulates the phosphorylation of some transcription factors such as the cAMP response element-binding (CREB), which controls the expression of DIO2 (13). This stimulation activates the type-2 5‘-iodothyronine deiodinase (DIO2), catalyzing the conversion of thyroxin (T4) to T3 in the BAT. T3 acts by increasing the capacity of cells depending on its α and β nuclear receptors, respectively, increases, leading to the activation of UCP1 and thyroid receptors.
UCP1, previously referred to as thermogenin, is responsible for the conductance of protons in brown adipocytes. Based on previous studies, the regulation mechanisms of UCP1 are mainly focused on these three pathways: the AMPK-SIRT1-PGC-1α axis, PRDM16, and the PGC-1α-PPAR complex. The master regulator of adipocyte differentiation PPARγ is necessary for the regulation of brown adipocyte-specific characteristics of adipocytes. Upon binding to its receptor, PPARγ promotes the expression of genes such as C/EBP, which stimulates the differentiation of brown adipocytes and links to PRDM16 (14). Subsequently, the coactivator 1α (PGC-1α) of PPARγ can be activated along with the enhancement of UCP1 expression (14). The main role of PGC-1α is to regulate energy balance by enhancing respiratory chain proteins and the expression of UCP1. On the other hand, PGC-1α-PPARγ activity seems to be run by PRDM16, which directly interferes with the transcription of thermogenic proteins (UCP1, DIO2, and PGC-1α), being essential in the promotion of the switch from white pre-adipocytes to brown adipocytes (15). Finally, regarding the AMPK-SIRT1-PGC-1α axis, the mechanism by which some ligands of PPARγ induce the transcription of genes involved in the process of WAT browning includes the activation of sirtuin 1 (SIRT1).
The SIRT family is one of the notably increased thermogenic and mitochondrial 363 factors. In the regulation of energy metabolism, SIRT1 is involved with several transcription factors for the control and function of mitochondrial biogenesis in brown adipocytes. In the muscle, SIRT1 controls the expression of AMPK whereas in hepatocytes it deacetylates PGC-1α (16). AMPK, as a well-recognized energy sensor, plays an important role in the regulation of cellular energy homeostasis. AMPK activation promots both BAT thermogenesis and the WAT browning process, whereas AMPK ablation results in cold intolerance and a reduction in non-shivering thermogenesis in mouse adipocytes. In relation to AMPK stimulation, SIRT1 is required for muscle mitochondrial function, described as decreased mitochondrial size and oxidation capacity. It is also known that thermogenic genes, such as those encoding for PGC-1α and UCP1, are known to be related to SIRT1 activation. Previous studies documented that overexpression of SIRT1 in the muscle downregulated PGC-1α levels, and while the AMPK activator AICAR increased the expression of PGC-1α, it also decreased the expression of SIRT1. It is therefore clear that elevated AMPK levels may influence PGC-1α activity in a manner that is largely dependent on SIRT1, which exerts a multiple effector-mediated influence on mitochondrial functions and WAT browning.
Besides transcriptional regulation, many other endocrine and locally secreted factors originating in different organs and involved in the regulation of thermogenesis and the development of brown adipose tissue have been discovered. Irisin is a PGC-1α-dependent myokine cleaved from fibronectin type-III domain-containing protein 5 (FNDC5) in direct relation to metabolism regulation. The stimulation of irisin expression in primary subcutaneous white adipocytes increases oxygen consumption together with an induction of UCP1 mRNA and other brown fat-related genes, which sheds new light on irisin's role in the development of brown adipocytes (17). In this regard, a positive correlation between the levels of UCP1 expression and the basal levels of brown gene expression in subcutaneous WAT in response to irisin has been described. Additionally, two important adipokines, leptin and FGF21, involved in regulating the browning process, act in an autocrine/paracrine manner to promote energy expenditure induced by irisin. Leptin crosses the blood-brain barrier affecting the feeding behavior and energy balance in the hypothalamus, including the dorsomedial hypothalamus (DMN), ventromedial hypothalamus (VMN), and ARC (18). Accordingly, mice deficient in FGF21 exhibited an impaired ability to adapt to chronic cold exposure, and decreased adaptive thermogenesis in BAT (19). All these processes are mediated by local (via induction of the PGC-1α protein) and central (via sympathetic activation) mechanisms.
WAT BROWNING AND OBESITY
Based on the great plasticity of adipose tissue, inducing WAT browning and activating BAT has become a potential therapeutic target for counteracting obesity by dissipating chemical energy as heat. The phenotype of WAT browning, which represents a particularly intriguing concept, involves several mechanisms in humans, and exerts a significant influence on thermogenesis, responsible for whole-body energy balance via activation of the sympathetic nervous system and/or via secretion of humoral factors (20). Accordingly, the identification of molecular pathways to manipulate the WAT browning process has gained great interest, as they may serve as a novel therapy in the treatment of obesity and associated metabolic disorders.
It is unsurprising that participants with abundant BAT had an increased energy expenditure and a lower body mass index (BMI) in a clinical trial. A human experimental research using single-timepoint infrared thermography also evaluated the relationship between BAT and BMI, which additionally confirmed the key role of WAT browning in obesity (21). A large retrospective cohort study demonstrated that BAT activity was negatively associated with visceral adipose tissue and, particularly, with BMI among 4,852 participants who underwent computed tomography scans and diagnostic positron-emission tomography (PET/CT) (22). Those participants with higher levels of BAT had less content of WAT in their subcutaneous adipose tissue and visceral adipose tissue. In subsequent studies, individuals with morbid obesity showed a positive correlation between active BAT and non-shivering thermogenesis after 1-year post bariatric surgery (23). Additionally, we observed that the non-obese mice had a significantly smaller size of adipocytes, mitochondria appearance around lipid droplets, increased mitochondrial density, high levels of UCP1, and WAT browning in an experimental study, all of which obese mice had not (23). This is also supported by the findings observed in obese subjects after interventional studies, in whom the thermogenic function of BAT contributed to body weight reduction (24).
Obesity is also a chronic low-grade inflammation of adipose tissue associated with increased circulating and local levels of pro-inflammatory cytokines, which may negatively affect the development of brown adipose tissue. Interestingly, recent reports have identified the adipocyte-expressed apoptosis signal-regulating kinase 1 (ASK1) as a regulator of WAT browning (25). Importantly, ASK1 signaling can be specifically activated by TNFα and the endotoxin lipopolysaccharide (LPS), resulting in elevated ASK1 activity. In obese human subjects, ASK1 levels were elevated in subcutaneous adipose tissue and found to be a negative modulator of WAT browning, thereby affecting body mass, glucose metabolism, and energy expenditure (26). Moreover, ASK1 depletion in mice fed a high-fat diet reduced body weight, improved glucose metabolism, increased energy expenditure, and induced browning of inguinal WAT, underscoring the potential importance of this inflammatory factor to combat obesity and other associated metabolic disorders (25). Conversely, chow-fed mice with overexpression of adipocyte specific ASK1 blunted browning of inguinal adipose tissue under cold exposure (25).
Numerous potential molecular mechanisms underlying this browning phenomenon have been identified in mice and human assays assessing the ability of candidate molecules to promote energy metabolism and thereby combat obesity, such as the chronic activation of nuclear factor κB (NF-κB) cascade, and increase mitochondrial thermogenesis activity in skeletal muscle. In summary, understanding the regulation pathways involved in BAT activation and WAT browning allowed the identification of some critical transcriptional regulatory factors and steps during both processes, and therefore many important therapeutic strategies for the future.
INTERVENTIONAL APPROACHES TARGETING WAT BROWNING
COLD EXPOSURE
Cold exposure is one of the oldest and most effective ways of inducing WAT browning without eliciting negative cardiovascular effects. Early studies in rodents have demonstrated that both chronic and acute cold exposure stimulated BAT insulin signaling, increased oxygen consumption, and also resulted in the emergence of UCP1-positive cells in mouse parametrial fat pad (26). Accordingly, prolonged exposure to cold at +4 ºC leads to an increase in UCP1 expression in experimental animals. The sensation of cold is acquired by skin receptors that convey signals via transient receptor potential (TRP) channels, which finally leads to stimulation of the peripheral sympathetic nervous system and BAT activation. The density of sympathetic innervation in WAT relies on its anatomical location, and chronic exposure to lower temperatures increases the number of sympathetic noradrenergic nerve fibers, which are strongly linked to the development of brown adipocytes.
There is also evidence from human assays that the levels of several hormones (including norepinephrine, ghrelin, and leptin) through a neural circuitry induced by cold exposure leads to a sympathetic outflow to BAT that turns on a thermogenic gene program defining a BAT-like molecular phenotype. Classically, NE, which is released from the sympathetic nerve terminal, can induce the production of FFA for UCP1 activation and lipolysis, and activate the BAT thermogenic program through PKA and p38 MAPK signaling. Recent studies have elucidated that cold exposure promoted a high production of eosinophil interleukin (IL)-4/13, resulting in the activation of M2 macrophages, which stimulated catecholamine production and tyrosine hydroxylase expression leading to browning of subcutaneous WAT (27). On the other hand, meteorin-like (Metrnl), which is a PGC1-α4-regulated hormone, also has an important role in the regulation of WAT browning by activating IL-4/13 and M2 macrophages upon cold exposure.
Moreover, cold exposure resulted in an increase of fatty acid uptake by BAT and oxidative metabolism even by 182 %, and of BAT volume by 45 % in humans. In a functional analysis of WAT and BAT, the results showed that BAT has a greater thermogenic capacity than WAT, while the molecular analysis of adipose tissue demonstrated the upregulation of genes only involved in the lipid metabolism of BAT as induced by cold exposure (28). It was estimated that more than two hours of very mild cold exposure could lead to a reduction of TG in BAT by about ~72 kcal (8 g of TG) with a mean total body BAT mass of 168 g (29). This phenomenon is associated with BAT thermogenesis, which drew attention to cold exposure targeting BAT activation. Together, these data indicate that cold exposure is a powerful stimulus targeting insulin sensitivity and lipid metabolism to promote WAT browning. The effects of cold exposure on WAT browning are shown in figure 1.
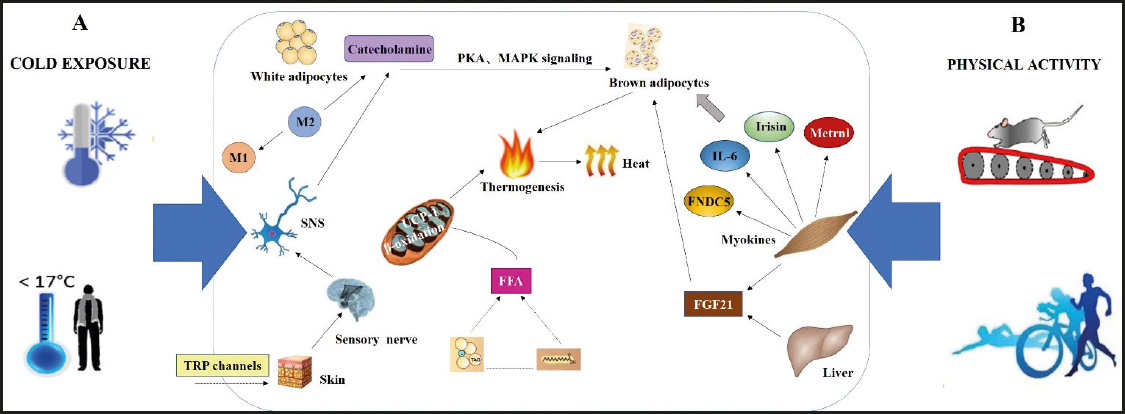
Figure 1. Effects of cold exposure and physical activity on WAT browning. Cold exposure and physical activity effects can cause the release of hormones and myokines, which act to induce WAT browning and increase energy expenditure in an endocrine and/or paracrine manner. A. Exposure to cold temperatures stimulates the peripheral sympathetic nervous system, resulting in the activation of M2 macrophages, which promotes catecholamine production and activates the BAT thermogenic program through PKA and MAPK signaling. Concurrently, NE, which is released from the sympathetic nerve terminal, induces the production of FFA for UCP1 activation and increases mitochondrial activity. B. Physical activity reduces adipocyte size, increases the number of beige adipocytes and elicits improvements in BAT activities via inducing the production of myokines secreted by skeletal muscle such as Metrnl, IL-6, irisin, and FNDC5, which could affect the thermogenic capacity of BAT. Additionally, physical activity also increases the synthesis of FGF21, which can result in a reduction of whole-body fat mass and an increase in brown adipocyte gene expression, accompanied by a marked enhancement of energy expenditure through the UCP1-mediated mitochondrial thermogenic pathway (M1: macrophages type 1; M2: macrophages type 2; SNS: sympathetic nervous system; PKA: protein kinase A; MAPK: AMP-activated protein kinase; IL-6: interleukin 6; FNDC5: fibronectin type III domain-containing protein 5; FGF21: fibroblast growth factor 21; FFA: free fatty acid).
PHYSICAL ACTIVITY
Exercise and physical activity play a significant role in the prevention of cardiometabolic diseases, eliciting several benefits on adipose tissues. More recently, it has emerged that factors induced during physical activity and secreted by peripheral organs such as the liver, adipose tissue, and potentially skeletal muscle act to induce WAT browning and increase energy expenditure in an endocrine and/or paracrine manner. The regulation mechanism that intervenes in this process is complex and influenced by many factors. Of these, Metrnl has also generated the most interest as an agent for WAT browning induced by PGC-1α4 after resistance exercise. Metrnl promotes the production of catecholamine and the activation of M2 macrophages from these cells, ultimately leading to BAT development and energy expenditure. During in vitro experiments, irisin has been shown to have browning capability in the subcutaneous WAT of mice, and upregulated levels of plasma irisin have been found in humans in response to exercise (30). On the contrary, serum analyses in obese individuals have shown a significantly elevated level of serum irisin (31). Numerous other molecular factors that are significantly involved in the browning process are also altered after chronic or acute endurance physical activities, including FGF21, β-aminoisobutyric acid, interleukin-6, and natriuretic peptides (NP's). However, most of these aforementioned factors have only been verified in rodents, and their functional effects still remain to be proven in humans.
FGF21 is an exercise-induced hormone shown to play a key role in the regulation of WAT browning. Administering FGF21 in vivo and in vitro stimulates the expression of brown fat-related genes such as UCP1 in inguinal and perirenal WAT. Moreover, the effects of FGF21 were found to be dependent on PGC-1α. In fat-specific PGC-1α knockout mice, the administration of FGF21 results in a reduction of whole-body fat mass and an increase in brown adipocyte gene expression, accompanied by a marked enhancement of energy expenditure and improvement of several metabolic indexes after physical activities (32). IL-6 represents another example of an exercise-induced myokine released by the muscles that has an influence on the differentiation of brown adipocytes. Lack of IL-6 diminishes the beneficial effects of thermogenic gene expression during prolonged exercise, suggesting that this myokine serves as an indispensable mediator of thermogenic functions in BAT (33).
Additionally, FNDC5, encoded by Fndc5, happens to act as an endocrine factor of WAT browning through yet unknown pathways. FNDC5 was identified as a myokine specifically regulated by PGC-1α and exercise, and its proteolytic cleavage can lead to the release of irisin. Consistent with these observations, researchers found a concomitant and FNDC5-dependent increase associated with WAT browning in myostatin-deficient mice, which have increased muscle mass (34). Translational studies published in recent years also showed a significant positive correlation between plasma levels of irisin and the expression of PGC-1α in exercise-induced muscle (35). Therefore, the role of FNDC5 and irisin in humans in terms of BAT activation and browning might be a great strategy for preventing and treating metabolic disorders. Additionally, the influence of exercise on WAT browning can be also attributed to increased mitochondrial activity in multiple tissues. Several animal studies have assessed the effects of exercise on mitochondrial activity and BAT thermogenesis, but resulting in mixed conclusions. Some studies demonstrated that exercise training increased mitochondrial respiration, UCP1 content, and mitochondrial activity, and induced gene expression for mitochondrial biogenesis in brown adipocytes (36). However, the results obtained by other studies showed that there is no direct correlation between exercise and both the mitochondrial and thermogenic activity of BAT (37). Together, these studies suggest that further research is needed to fully establish the role of physical activity on the thermogenic function of BAT. The effects of physical activity on WAT browning are shown in figure 1.
DIET
Data from experimental studies suggest that some dietary components can increase the metabolic rate via thermogenic effects by augmentation of BAT activity. The molecular mechanisms triggered by dietary components to promote thermogenic responses in brown adipocytes include, but are not limited to, activation of AMPK/SIRT1 and PPARγ-PRDM16 signaling pathway, modulation of thermogenic and anti-obesity adipokine secretion, and morphological and epigenetic changes. Several plant-based compounds and dietary factors such as capsaicin, curcumin, caffeine, catechins and resveratrols have been demonstrated to have significant effects on WAT browning based on both in vitro and in vivo studies. The summary of dietary components that induce WAT browning are shown in figure 2.
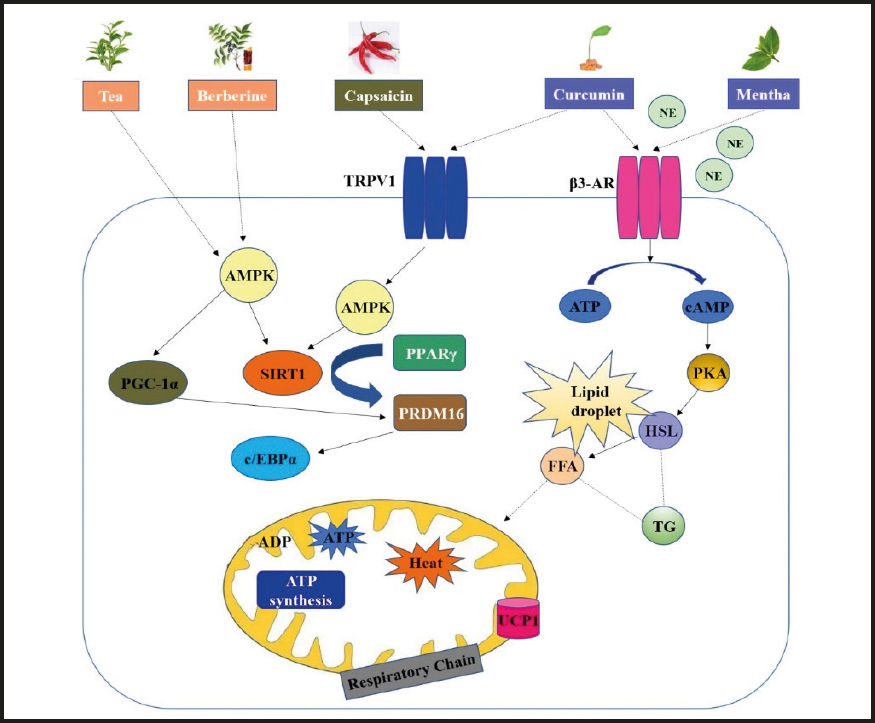
Figure 2. Summary of dietary components that induce WAT browning (TRPV1: transient receptor potential vanilloid 1; β3-AR: β3 adrenergic receptor; NE: norepinephrine; AMPK: AMP-activated protein kinase; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator alpha 1; SIRT1: sirtuin 1; PPARγ: peroxisome proliferator-activated factor gamma; PRDM16: PR domain containing 16; C/EBPβ: CCAAT/enhancer binding-proteins; UCP1: uncoupling protein 1; cAMP: cyclic adenosine monophosphate; HSL: hormone-sensitive lipase).
Capsaicin (and related capsinoids), as alkaloids present in plants belonging to the Capsicum genus, has been shown to induce WAT browning in mice via multiple different mechanisms, including the activation of gastrointestinal transient receptor potential vanilloid 1 (TRPV1) receptors, the modulation of the AMPK/SIRT1 pathway, and the modulation of PPARγ-PRDM16 interaction. In animal studies, the oral administration of capsaicin and/or capsinoids results in an increase in sympathetic nerve activity innervating WAT browning, and whole-body energy expenditure, and a decrease in body fat mass by stimulating TRPV1 expression in sensory nerves within the gastrointestinal tract (38). It was also reported that a lack of TRPV1 in mice can diminish the weight loss and thermogenic effects caused by capsinoids (39). It is possible that the thermogenic effects of capsinoids are directly subjected to the regulation of TRPV1 as expressed in BAT, thereby increasing energy expenditure. During in vitro studies capsaicin stimulated the phosphorylation of SIRT1 by activating AMPK and the expression of transcription factors PPARγ and PRDM16, finally leading to UCP1 synthesis accompanied by a subsequent activation of BAT thermogenesis and browning.
Recently, other representative dietary compounds that have an ability to induce BAT activation is polyunsaturated fatty acids (PUFAs), a polyphenol with protective effects against metabolic alterations such as obesity and dyslipidemias. In animal models, the influence of PUFAs on the expression of UCP1 mRNA in brown adipocytes or WAT browning can be affected by high-fat diets. The results from two other studies demonstrated that supplementation with diets rich in omega n-3, long-chain polyunsaturated fatty acids significantly promoted thermogenic effects by activating the brown adipose tissue (40,41). Furthermore, a maternal diet rich in polyunsaturated fatty acids also contributed to larger interscapular brown adipose tissue depots in animals (42). The underlying molecular mechanisms responsible for the thermogenic function of PUFA are controversial, including the activation of TRPV1, immediately stimulating ADRB3 but also free fatty acid receptor 4 (FFAR4), which ultimately resulted in the up-regulation of several miRNAs involved in adipocyte browning. However, it has to be further proved whether dietary n-3 PUFAs exert thermogenic effects in humans.
Curcumin is a polyphenol compound extracted from the rhizomes of the plant Curcuma longa, commonly known as turmeric, with anti-obesity and anti-hyperglycemic properties. A large number of studies have confirmed the hypothesis that the administration of curcumin, by suppressing inflammatory reactions in adipocytes and lipogenesis in the liver, may promote thermogenesis in BAT and/or the browning process in rodents. For instance, Wang et al. (43) reported that mice displayed a reduction of fat mass, body weight gain, and a better tolerance to cold exposure, without any effect of food intake, after the administration of 50 or 100 mg/kg/day of curcumin for 50 days. Particularly, the role of curcumin in the induction of WAT browning has also been described in these animals. In vitro studies have shown that curcumin induced mitochondrial biogenesis and WAT browning through an AMPK dependent pathway in 3T3-L1 cells and a primary culture of inguinal white adipocytes, and markedly drove the BAT thermogenic program by increasing several brown fat markers in both types of adipocyte in a dose-dependent manner (doses ranged from 1 to 20 µM) (44). Besides, a proteome analysis aiming at primary inguinal white adipocytes indicated that curcumin increased the expression of critical mitochondrial proteins responsible for lipolysis, browning-specific markers, and the oxidation of fatty acids (2). However, if curcumin induces WAT browning in humans has yet to be determined.
Other intriguing dietary compounds such as caffeine and catechins can also promote energy expenditure and BAT thermogenesis. Green tea is abundant in catechins, and has a high content of polyphenols, which have apparent thermogenic and anti-obesity properties. In humans, an oral administration of catechin-rich tea produced an acute increase in energy expenditure and activation of thermogenesis with higher BAT activities in a similar way in different doses (45). It has been reported that green tea extracts exert their thermogenic response through the direct activation of the AMPK cascade in BAT, including the overexpression of brown-specific and anti-adipogenic genes (46). In addition to the dietary compounds discussed above, various dietary components such as resveratrols, thyme, mentha, and berberine have revealed their thermogenic potential via multiple mechanisms of action during in vitro and animal studies. In the future, more clinical trials are necessary to evaluate their involvement in the browning process in humans.
THE CORE REGULATORS OF WHITE ADIPOSE TISSUE BROWNING INVOLVED IN OBESITY
The functional importance of brown adipose tissue for heat generation and energy consumption to protect animals from obesity and other metabolic disorders has been emphasized for decades and has become the new focus in the field of endocrinology in recent years. Understanding the molecular mechanisms responsible for WAT browning allowed the development and administration of new targeted approaches to intensify this process for the induction of energy consumption, the purpose of which is to tackle obesity and/or ameliorate the metabolic profile. Several representative transcription factors and co-regulators that affect WAT browning have sparked an interest in the possibility of further identifying molecular switches in the browning process. The selected molecules involved in white adipose tissue browning and brown adipose tissue activation that could exert potential therapeutic effects on obesity are shown in table II.
Table II. Selected molecules involved in white adipose tissue browning and brown adipose tissue activation that could exert potential therapeutic effects on obesity
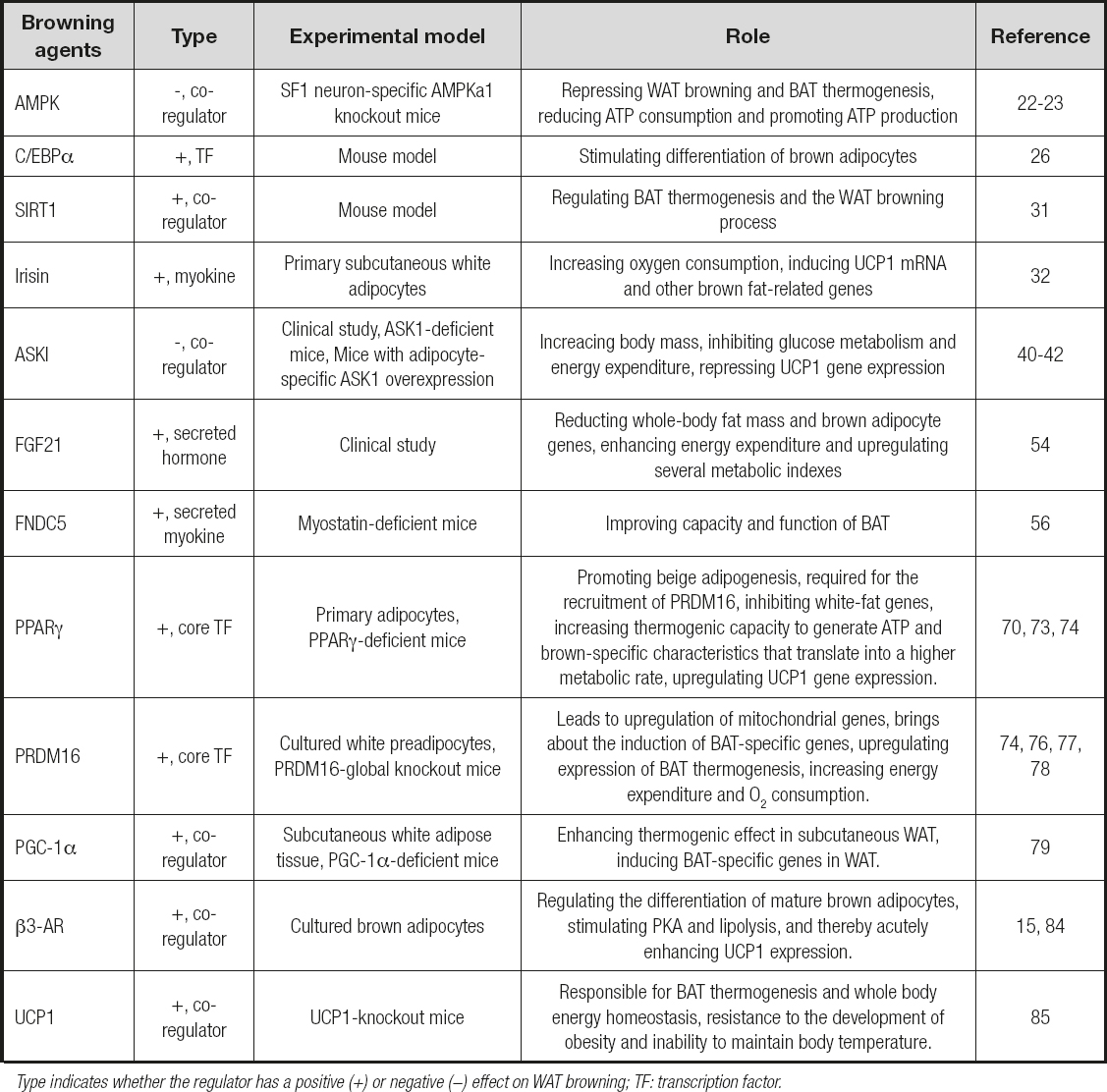
Type indicates whether the regulator has a positive (+) or negative (−) effect on WAT browning; TF: transcription factor.
ROLE OF PPARγ
Although different routes are followed by white and brown adipocytes during the differentiation process from mesenchymal stem cell to adipocyte, both of them share a similar transcriptional program. PPARγ is highly expressed in both adipocyte types, where it is a key regulator indispensable for adipocyte differentiation and survival. PPARγ belongs to a sub-family of the peroxisome proliferator-activated receptor family, of which the firstly identified PPARγ was able to regulate gene expression by binding to PPARγ Responsive Elements (PPREs) after dimerization with RXRs. Due to alternative promoter usage, PPARγ exists as two protein isoforms, PPARγ1 and PPARγ2 which differ in their N-terminal. Previous studies (47) have demonstrated that the PPARγ2 expression stimulated adipogenesis in cultured fibroblasts including the conversion of cell morphology, lipid accumulation, and expression of target genes closely associated with the adipocyte phenotype.
PPARγ is subjected to several post-translational modifications and interacts with various other transcriptional regulators. It was found that the expression of UCP1, which is a BAT hallmark gene responsible for thermogenesis, would be induced by PPARγ activator rosiglitazone in WAT of both mice and humans. Petrovic et al. (48) treated primary cultures of mouse epididymally-derived white adipocytes differentiated to mature adipocytes with a potent PPARγ agonist in vitro, and observed marked UCP1 gene expression in approximately 10 % of these pure white adipocyte cultures, as revealed by immunocytochemical staining. These cells not only increased the expression of UCP1, but also of Elovl3 (elongation of very long-chain fatty acids (FEN1/Elo2, SUR4/Elo3, yeast)-like 3), PGC-1α (peroxisome proliferator-activated receptor γ coactivator 1α), and other genes related to mitochondrial biogenesis. PPARγ activation can promote beige adipogenesis via PGC-1α, which plays a crucial role in oxidative metabolism, adaptive thermogenesis, and mitochondrial biogenesis. It has also been demonstrated that SIRT1-mediated deacetylation of PPARγ is required for the recruitment of PRDM16, which is the determinant factor of brown fat development and sufficient for the process of brown adipogenesis in WAT (49). In a chromatin immunoprecipitiation analysis combined with massive parallel sequencing (ChIP-seq) to identify genome-wide PPARγ binding sites in epididymal WAT and primary interscapular BAT, early B-cell factor-2 (Ebf2) has been shown to regulate PPARγ binding activity, which could determine adipocyte identity (50). When Ebf2 is expressed in white pre-adipocytes or myoblasts, PPARγ binding to its brown-selective sites and reprogrammed cells increase. Consistently, brown adipocytes and tissues in PPARγ-deficient mice have displayed a loss of thermogenic capacity to generate ATP and brown-specific characteristics that translates to a lower metabolic rate (51). Several other genes specialized in WAT browning are involved in the process of adipogenic differentiation, many of which contain functional PPARγ response elements such as aP2.
Furthermore, PPARγ was shown to be not only involved in the induction of brown fat genes but also the inhibition of white fat genes during the browning process. For example, the troglitazone-associated inhibition of white fat genes will be prevented in 3T3-L1 adipocytes when mutation has occurred at the PPARγ ligand binding site (51). The interplay of the regulators described above highlights the key role of PPARγ in the browning process, which involves a series of transcription activators and repressors.
ROLE OF PRDM16
PRDM16 is a zinc finger protein that was identified as a brown fat-selective cofactor able to activate the brown adipose differentiation programs. Quantitative analyses evaluating the expression of PRDM16 at the mRNA level demonstrated a 15-fold enrichment in brown adipose tissue relative to white adipose tissue (52). Importantly, PRDM16 mRNA expression increased 20-fold during the differentiation of brown adipocytes in a culture (52). Overexpression of PRDM16 in cultured white preadipocytes will lead to an up-regulation of mitochondrial genes, accompanied by an increase in uncoupled respiration and mitochondrial biogenesis, hallmarks of BAT. These data highly suggest that PRDM16 serves as a determining factor to promote WAT browning in subcutaneous WAT. In a follow-up study, PRDM16 has been demonstrated to be a cell fate switch, which determines myf5 positive precursor cells to become brown adipocytes or skeletal myoblasts, both of which are derived from the same cell type (53). PRDM16 has also been shown to be required for the enhancement of the transcriptional activity of PPARγ by binding to PPARγ in a ligand-independent manner, indicating that PPARγ activation plays an important role in the adipogenic function of PRDM16.
Mechanistically, it has also been confirmed that PRDM16 could bring about the induction of BAT-specific genes such as UCP1 by directly interacting with PGC-1α and PGC-1β. The late-stage embryos (E17) of PRDM16 global knockout mice show that BAT was severely affected accompanied simultaneously by increased expression of skeletal myogenic genes, and reduced expression of BAT thermogenic and selective genes, supporting a role of PRDM16 as a negative regulator of skeletal muscle development and an early determinant of the brown fat lineage (53). An analysis of transgenic mice with in vivo overexpression of PRDM16 in white fat, induced by a high-fat diet, demonstrated that the molecular changes present were associated with reduced energy accumulation and increased energy expenditure, protecting the whole body from the weight gain, all of which indicated selective effects of PRDM16 in adaptive thermogenic responses (54). In addition, mice with specific ablation of PRDM16 in adipose tissues showed significant reductions in O2 consumption and thermogenic gene expression of white adipose tissue both in the basal state and following stimulation under cold exposure, indicating that PRDM16 is required for the browning of white adipose tissue and the healthy effects of subcutaneous adipose tissue (55). However, the effects of PRDM16 ablation appeared to be inguinal fat-specific, altering the function of beige adipose tissue but not causing depletion in brown adipose tissue. In general, these loss- and gain-of-function researches, performed both in vitro and in vivo, have confirmed the crucial role of PRDM16 in the development of brown adipose tissue and related regulatory programs.
Besides, several other genes that were not previously known to be expressed selectively in white versus brown adipocytes, such as neurotrophic tyrosine kinase receptor type 3 (ntrk3), otopetrin-1 (otop1) and epithelial like antigen-1 (eva1), were significantly induced by PRDM16 expression. Moreover, the expression of several important thermogenic genes was increased in PRDM16-transduced cells via a cAMP-dependent manner, and the mRNA levels of PGC-1α, UCP1, and deiodinase-d2 were elevated to very high levels by PRDM16 in response to cAMP. On the other hand, PRDM16 expression also has a great influence on the mRNA levels of those genes related to mitochondrial oxidative phosphorylation, such as cox4i1, cox5b, and cytochrome c (cyc), that are abundant in brown adipocytes. Altogether, these results strongly suggest a role for PRDM16 as a positive regulator of the WAT browning program.
ROLE OF PGC-1α
PGC-1α was first characterized in the late 1990s after the identification of novel interactors of the master regulator of adipocyte differentiation, PPARγ, in brown adipose tissue, which was performed in a yeast two-hybrid screen. It has now been recognized that PGC-1α can alter the transcriptional activities of a number of key mitochondrial genes encoding for proteins involved in metabolic functions, which ultimately led to an increase in mitochondrial DNA. The expression of PGC-1α is induced upon cold exposure in brown adipose tissue. Once activated, PGC-1α stimulates the transcriptional networks that control the oxidative phosphorylation and mitochondrial biogenesis of energy substrates, leading to the initiation of tissue-specific gene programs that adjust hepatic gluconeogenesis, lipid metabolism, and thermogenesis in the brown adipose tissue. Remarkably, mice with adipose PGC-1α deficiency are cold-sensitive owing to thermogenic dysfunction, mainly caused by diminished UCP1 expression, impaired fatty-acid β-oxidation, and also electron transportation.
In primary human subcutaneous white adipose tissue, adenovirus-mediated expression of PGC-1α stimulates the phenotype of brown adipocytes, accompanied by increased expression of UCP1, fatty acid oxidation enzymes, and respiratory chain proteins. Mice deficient in PGC-1α underwent a blunted expression of mitochondrial genes and diminished thermogenic effects in subcutaneous WAT. However, the physiological role of PGC-1α in a different adipose tissue-specific PGC-1α-deficiency mouse model has been demonstrated to be dispensable for the management of mitochondrial biogenesis and respiration through the regulation of nuclear respiratory factors and the induction of uncoupling proteins, even though it plays an important role for the induction of BAT-specific genes such as UCP1 in subcutaneous WAT (56). The analysis by immunohistochemistry of WAT reveals the presence of UCP1-positive multilocular cells, which are seen as a sign of browning. This suggests that there is cross-talk between PGC-1α and the WAT browning process. This is in line with the definition of PGC-1α as a cold-induced co-activator of thermogenesis under the control of mitochondrial gene expression.
In addition to the classic factors involved in brown adipocyte functions, other inducers such as CREB signaling and β-adrenergic stimuli are known to regulate PGC-1α mRNAs. FGF21 has emerged as an important regulator in this thermogenic recruitment of WAT by regulating PGC-1α protein levels in an autocrine/paracrine fashion, finally prompting PGC-1α-dependent WAT browning. Moreover, it has been demonstrated that the expression of PGC-1α protein can be regulated by factors such as pRb (retinoblastoma protein) and the Rb family member p107, prompting PGC-1α-dependent WAT browning (57). Interestingly, BAT-like features in WAT of p107-knockout mice were displayed with elevated PGC-1α and UCP1 expression, while pRb is significantly reduced in p107-knockout mice. As repressive effects of pRb on PGC-1α expression by binding to its promoter, both pRb and p107 are likely to exert a negative effect on the browning process because of their interaction with PGC-1α. To conclude, PGC-1α is known as a critical transcriptional co-regulator for mitochondrial oxidative metabolism and hence for WAT browning. A great emphasis has been placed on the identification of the signaling pathways activating PGC-1α and its related downstream targets that can ultimately enhance a negative energy balance.
ROLE OF UCP1
UCP1, which resides within the inner mitochondrial membrane, was first isolated from the BAT of cold-exposed rats 40 years ago and originally termed thermogenin for its heat-producing function in non-shivering thermogenesis. UCP1 is known as a brown adipocyte-specific natural, regulated uncoupler of mitochondrial respiration: its activity translates the high oxidation rates of brown adipocytes into heat production to defend core body temperature under cold exposure by dissipating the energy proton gradient from the respiratory chain after the facilitation of proton reentry into the mitochondrial matrix. During cellular respiration, the uncoupling of oxidative phosphorylation represents about 50 % of energy expenditure from the mitochondria of a cell with normal function. Taking the laws of thermodynamics into consideration, most of the energy that is generated by the electrochemical potential of the oxidation process in brown adipocytes is not used for the phosphorylation of ADP, but is dissipated as heat. It is estimated that 60 g of BAT could contribute to more than 20 % of heat generation under controlled cold conditions in the human body.
There is considerable evidence that UCP1 is mainly responsible for BAT thermogenesis and whole-body energy homeostasis. The expression of the UCP1 gene can be activated by thyroid hormones, b3-agonists, cold exposure, cAMP, and adrenergic stimulation, and inhibited by purine nucleotides (ADP and ATP). In previous studies, the sympathetic nervous system was recognized and confirmed as the main trigger of UCP1 induction and activation based on the use of drugs that activate β3-ARs. In addition, the uncoupling of the mitochondrial respiratory chain induced by UCP1 action takes place only after a proper stimulation of cells by norepinephrine. In the basal status, the proton-translocating activity of UCP1 is blocked by the binding of purine nucleotide di-and tri-phosphates at its outer facing cavity. In response to a cold environment or nutrients, norepinephrine is released from the sympathetic circuits onto β3-ARs to activate thermogenesis in thermogenic adipocytes. Subsequently, β3-AR signaling will stimulate PKA and lipolysis by elevating cAMP levels, thereby acutely enhancing UCP1 expression through multiple transcriptional and post-transcriptional mechanisms directly induced by free fatty acids. This finding indicates the existence of thermogenesis effects is physiologically relevant to UCP1 in adipocytes. Accordingly, the potential sites for the induction of brown adipocytes and the regulation of UCP1 are multifarious, and many of the transcription factors and molecules of the signaling pathway are involved in various aspects of adipocyte biology, as well as in muscle structure and function. More recently a proof-of-principle study showed that UCP1-knockout mice at thermoneutrality were slightly prone to diet-induced obesity and resistant to obesity when housed at room temperature, suggesting a central role of a UCP1-independent thermogenic pathway in counteracting the development of obesity in mice (58). Thus, reduced lipid deposition, greater thermogenesis, and higher UCP1 expression in BAT could be helpful for adequate body weight control.
CONCLUSIONS AND FUTURE PERSPECTIVES
Over the last decade, white adipose tissue browning has garnered great interest as a new therapeutic strategy to combat obesity, as numerous studies have established an association between BAT activities and energy balance. In recent years, various research studies both at home and abroad have significantly increased our understanding of the regulation mechanisms related to the process of WAT browning. Currently, the best-explored activators of WAT browning include cold exposure, physical activity, and diet, which have been known for years. Many critical transcription molecules, secreted factors, and critical steps have been implicated in the browning process. It is interesting that a majority of these molecules exert their effects through a series of cascade molecular reactions including binding, interacting, inhibiting, or activating the primary transcriptional regulators during the development of traditional brown adipose tissue: PPARγ, PRDM16, PGC-1α, and UCP1. The metabolic activation of brown adipose tissue does not only promote energy expenditure by regulating BAT thermogenesis, but also serves as an effective adjunct therapeutic target for metabolic complications such as obesity, insulin resistance, and disturbances in glycolipid homeostasis. In terms of the metabolic advantages resulting from the initiation of WAT browning, the most important future perspective will focus on exploring new molecular factors and related signaling mechanisms, thus elucidating WAT browning as well as BAT activation.
Since the recruitment and activation of BAT in obese individuals plays an especially important role for promoting a negative energy balance, the critical issue with regard to the relationship between inactive BAT and energy metabolism under the obesity condition needs to be further addressed. An increasing number of studies in mouse models as well as in vitro models of cell lines point to WAT browning strategies aiming at diet-induced and/or genetically determined obesity. Unfortunately, this hypothesis has not been confirmed in an in vivo model by inducing BAT activation and WAT browning in humans. It remains challenging to identify BAT activators that will still work properly under other conditions other than cold exposure, physical exercise, and diet. Moreover, there is still controversy over the issue of how to activate and maintain a significant number of brown adipocytes for anti-obesity effects. Another major goal for future research is a clarification of brown adipokines or batokines, which probably could be candidates for drug development to combat obesity and related metabolic disorders.