INTRODUCTION
Nowadays, diabetes mellitus has gradually become an increasingly striking social health problem around the world. In 2017, the eighth edition of the International Diabetes Federation (IDF) Diabetes Atlas showed that there were about 425 million diabetics worldwide and the number may increase to 700 million by 2045 (1). The incidence of diabetes mellitus (DM) in China is about 10 % (2), and the number has reached 114 million, amounting to 1/3 of the total of global diabetics. T2DM accounts for more than 90 % of total diabetes patients (3,4).
The intestinal flora is to date considered a complex organ composed of 500-1000 species and 1014 bacteria, which is more than 10 times the number of human cells, including bacteria, viruses, fungi, and protozoa, that are commensal with the human intestinal tract (5). Among these, bacteria represent the best studied group and will be the main focus of this review. Overall the predominant bacterial groups in the microbiome are Gram-positive Firmicutes and Gram-negative Bacteroidetes (6,7). Studies have shown that lots of chronic diseases may be related to intestinal microecological disorders (8), that intestinal flora is an important factor among environmental factors, and that its changes are related to a series of metabolic diseases such as obesity and DM (9,10).
At the same time, a lot of factors can contribute to changes in intestinal flora. Firstly, it is now understood that diet plays a significant role in shaping the microbiome, with experiments showing that dietary alterations can induce large, temporary microbial shifts within 24 h. The gut microbiota of patients with type-2 diabetes has been functionally characterized with diabetes-associated markers, showing enriched membrane transport of sugars and branched-chain amino acids, xenobiotic metabolism, and sulfate reduction along with decreased bacterial chemotaxis, butyrate synthesis, and metabolism of cofactors and vitamins (11). Revealed by metagenome analysis, gut microbiota composition transforms through early stages of human development, and is influenced by the diet (12). Secondly, regular exercise has an anti-inflammatory effect, which improves the immunological profile in type-2 diabetes mellitus (13). Thirdly, the intestinal flora changes with aging. Besides these, some other factors, such as drugs (16-17), lifestyle (30), etc., also play a role in the development of intestinal flora.
It is of huge importance to fully understand the changes of intestinal flora in T2DM, and the pathological mechanism of involvement of intestinal flora in the development of T2DM. Similarly, an in-depth study of risk factors associated with bacterial reduction in patients with T2DM is needed, including the impact of exercise, food, specific diets, drugs and probiotics. In this article, we carried out a review based on several published clinical trials to determine the correlation between T2DM and intestinal flora. We hope that this article may provide a theoretical basis and literature references for the regulation of intestinal flora in the treatment of T2DM and its complications.
MATERIALS AND METHODS
IDENTIFICATION OF ELIGIBLE STUDIES
One search strategy was run using "Intestinal flora" and "Type 2 diabetes mellitus" with no limitations. Besides, another search strategy was also run using the terms "Intestinal flora" and "Type 2 diabetes mellitus" limited to "humans" and "clinical trial". A broad search of the English-language literature for randomized controlled trials (RCTs) in patients with T2DM was performed by using Cochrane, Medline, PubMed, Central Register of Controlled Trials, Web of Science, and trial registry databases. All the relevant publications were reviewed, and duplications of articles from the two search strategies were avoided. The articles in reference lists were also hand-searched for potentially relevant publications. The search was conducted by two investigators. Any disagreements were resolved by consensus with involvement of the third author.
INCLUSION AND EXCLUSION CRITERIA
Human-associated studies, regardless of year of publication, would be included if they met the following criteria: 1) RCT; 2) patients had been diagnosed with T2DM; 3) were older than 18 years; 4) sufficient data were available of clinical outcomes. Studies would be excluded if they met the following criteria: animal experiment, review, mechanism research, case report, collection of papers, literature with incomplete data and/or duplicates, and no full manuscript availability. Participants would be excluded if they 1) had severe conditions, including digestive dysfunction, heart failure, renal failure, malignant tumors, severe cerebrovascular diseases, ketosis, hyperthyroidism, liver dysfunction, or severe gallbladder and pancreatic diseases; 2) were pregnant or lactating; 3) suffered from mental illness; 4) used anti-depressants, sedatives, neurological or psychiatric medications; 5) required insulin therapy or had arterial hypertension and dyslipidemia controlled by statins and either ace-inhibitors or angiotensin receptor blockers; 6) had diabetes-specific complications and ischemic heart disease; 7) lacked the ability to perform physical activities.
DATA EXTRACTION
Two investigators extracted the data independently and reached a consensus on all items. For each study, the following information was collected: first author, year of publication, sample size, mean age, gender, anthropometric measurements (weight, height, body mass index (BMI), waist circumference, and body composition by bioelectrical impedance analysis), dietary record, trial duration, and clinical outcomes. Clinical outcomes included: glycated hemoglobin (HbA1c), low-density lipoprotein-cholesterol (LDL), high-density lipoprotein-cholesterol (HDL), cholesterol (CHOL), triglycerides (TG), aspartate aminotransferase (AST) and alanine transaminase (ALT), free fatty acids, CRP, homeostasis model assessment of insulin resistance (HOMA-IR), biochemical analyses, fasting GLP-1 concentration, and gut microbiota. Data could be extracted separately as long as there was enough information available in the trials.
RESULTS
LITERATURE SEARCH
We used the terms "Intestinal flora" and "Type 2 diabetes mellitus" with a broad search for randomized controlled trials (RCTs) in patients with T2DM. The results: Cochrane (700), Medline (733), PubMed (711), Central Register of Controlled Trials (688), Web of Science (699). In the end, a total of 744 articles (all published) were retrieved from the databases. A total of 360 articles about animal experiments, 262 articles with reviews, 9 articles on meta-analyses, 3 articles in letter format, 1 case report, 15 articles without full text, and 69 articles about non-randomized controlled trials were excluded, and 12 articles with incomplete data were also excluded. Thus, a total of 13 publications met the inclusion and exclusion criteria, and details from these trials were extracted separately. Figure 1 shows a flowchart of article selection and inclusion. Because of the heterogeneity found among patients and trial methods, and the large variety of outcome measurements used in these trials, the pooling of data for a meta-analysis was inappropriate. Results were, therefore, summarized qualitatively.
STUDY CHARACTERISTICS
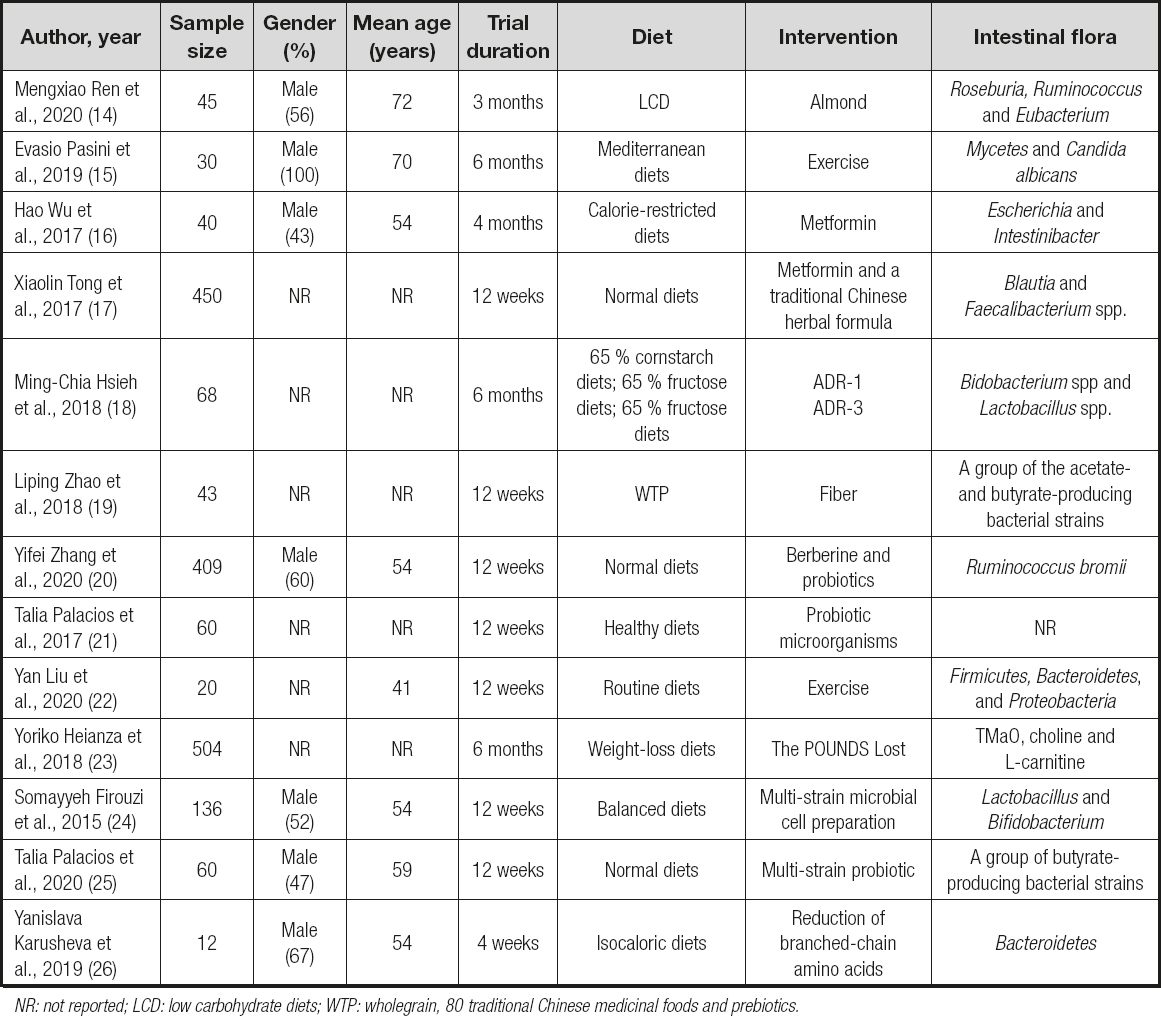
Details from 13 eligible published trials are discussed in table I. Table I summarizes the characteristics of the 13 trials. The number of participants in these trials ranged from 12 to 504, with median age ranging from 41 to 72 years. Trial duration ranged from 1 to 6 months, and intervention included exercise, food, specific diets, drugs, and probiotics. The intestinal flora of all patients in our study were classified in categories.
CLINICAL OUTCOMES
Effect of exercise on intestinal flora in diabetes
A total of 2 trials in table II demonstrated that the exercise group had shown significant improvements of lean mass from baseline amomg diabetes patients. Otherwise, fat mass and fasting glucose had decreased obviously. According to Evasio Pasini et al. (15), those who received exercise training had improved blood sugar, as well as functional and anthropometric variables. Moreover, chronic exercise reduced intestinal fungus overgrowth, leaky gut, and systemic inflammation. As well, in the research by Yan Liu et al. (22), the microbiome of responders exhibited an enhanced capacity for biosynthesis of short-chain fatty acids and catabolism of branched-chain amino acids.
Effect of food and specific diets on intestinal flora in diabetes
A total of 3 trials involved food and specific diets with effects measured on intestinal flora (Table III). They improved HbA1c (p < 0.01) and helped with weight loss. In the study of Mengxiao Ren et al. (14), A-LCD significantly increased the short-chain fatty acid (SCFAs)-producing bacteria Roseburia, Ruminococcus and Eubacterium. Liping Zhao et al. (19) showed that a select group of SCFA-producing strains was promoted by dietary fibers and that most other potential producers were either diminished or unchanged in patients with T2DM. However, in the trial by Yanislava Karusheva et al. (26), the oral glucose sensitivity index was 24 % (p < 0.01) and circulating growth factor 21 was 21 % higher (p < 0.05), whereas meal-derived insulin secretion was 28 % lower (p < 0.05). Then it could be seen that the effect of food and specific diets was obvious in improving glucose in diabetics, and may impact some pathological mechanisms.
Effect of drugs on intestinal flora in diabetes
In the work by Hao Wu et al. (16), their findings provide support for the notion that an altered gut microbiota mediates some of metformin's antidiabetic effects. In the study of Xiaolin Tong et al. (17), they also found that both metformin and AMC significantly alleviated hyperglycemia and shifted gut microbiota structure in diabetic patients.
They significantly increased a co-abundant group represented by Blautiaspp, which significantly correlated with the improvements in glucose. Yifei Zhang et al. (20) measured alterations in gut microbiota using oral intake of probiotics or berberine (BBR), a bacteriostatic agent. All 3 trials demonstrated drugs had an impact on intestinal flora (Table IV). The three studies suggested that after the intervention with drugs, body weight loss and HOMA improve along with changes in intestinal flora.
Effect of probiotics on intestinal flora in diabetes
The intestinal flora was significantly changed in diabetic patients after an intervention with Lactobacillus ADR-1 or ADR-3. According to Ming-Chia Hsieh et al. (18), the consumption of different strains of L. reuteri may influence changes in intestinal flora, which may lead to different outcomes after probiotic intake (Table V). In the study by Talia Palacios et al. (21), intentional manipulation of gastrointestinal microbial profiles may be useful for preventing and controlling type-2 diabetes mellitus and its associated metabolic complications. Somayyeh Firouzi et al. (24) proved that probiotics can also modestly improve HbA1c and fasting insulin in people with type-2 diabetes. Besides, Talia Palacios et al. (25) suggested that probiotics may act as an adjunctive to metformin by increasing the production of butyrate, which may consequently enhance glucose management.
Effect of other methods on intestinal flora in diabetes
In the study by Yoriko Heianza et al. (23), they found the importance of changes in TMaO, choline and l-carnitine in improving insulin sensitivity during a weight-loss intervention for obese patients. Dietary fat intake may modify the associations of TMaO with insulin sensitivity and glucose metabolism.
SIDE EFFECTS
In these studies, more participants experienced gastrointestinal AEs in the treated groups, although the AEs did not affect the antidiabetic effect or gut microbiome features related to interventions in these studies. Again, this concern needs to be addressed in trials with longer intervention durations. Although the consumption of live probiotics products is generally considered safe for most human beings, some side effects have been reported under certain conditions. For example, live probiotics may become pathogenic when used in subjects with severe immune deficiency (27). In infants with short bowel or cardiac stenosis, bacteremia has been reported in some cases (28).
DISCUSSION
Research evidence from human intestinal microbial profiles demonstrates that each individual has a unique intestinal bacterial composition (in diversity and abundance). Modern pharmacological studies have shown that intestinal flora plays an important role in the occurrence and development of T2DM, ditto that for genetic, environmental, and dietary factors (29,30). However, the differences in intestinal flora may be due to different ethnicity, age, and gender. In addition, the mechanisms responsible for ecological imbalance, as well as the mechanistic link between altered gut flora and diabetes, is not yet clear.
EXERCISE AND INTESTINAL FLORA IN DIABETES
Exercise increased lean mass, reduced fat mass and inflammation with better glycemic control and physical performance. Some experimental research shows that physical activity may modify fecal short-chain fatty acids, which increases the presence of fecal butyrate and in turn butyrate-producer intestinal bacteria (31). Short-chain fatty acids activate muscular AMPK, an enzyme that regulates muscle metabolism of glucose and lipids. These metabolic effects may be important in diabetes and confirm cross-communication between microbiota, exercise, and global metabolism (32). And they suggest a link between microbiota, muscle, the brain, and human metabolism. The cure of intestinal microbiota with physical exercise and/or specific therapies could be an important step for tailored therapy allowing traditional therapy and global patient metabolism to function properly.
FOOD, SPECIFIC DIETS AND INTESTINAL FLORA IN DIABETES
An acute change in diet - for instance, one that is strictly animal-based or plant-based - alters microbial composition within just 24 h of initiation, with reversion to baseline within 48 h of diet discontinuation (33). Furthermore, the gut microbiome of animals fed a high-fat or high-sugar diet is more prone to circadian rhythm disruption (34). Studies also suggest that overwhelming systemic stress and inflammation - such as that induced via severe burn injury - can also produce characteristic acute changes in the gut microbiota within just one day of the sustained insult (35).
Several popular diets, including western, gluten-free, omnivore, vegetarian, vegan, and Mediterranean, have been studied for their ability to modulate the intestinal microbiota. In several studies, a western diet (high in animal protein and fat, low in fiber) led to a marked decrease in numbers of total bacteria and beneficial Bidobacterium and Eubacterium species (36-38).
Across the spectrum, the Mediterranean diet is highly regarded as a healthy balanced diet. It is distinguished by a beneficial fatty acid profile that is rich in both monounsaturated and polyunsaturated fatty acids, high levels of polyphenols and other antioxidants, high intake of fiber and other low glycemic carbohydrates, and relatively greater vegetable versus animal protein intake. Specifically, olive oil, assorted fruits, vegetables, cereals, legumes, and nuts, moderate consumption of fish, poultry, and red wine; and a lower intake of dairy products, red meat, processed meat and sweets characterize the traditional Mediterranean diet (39).
DRUGS AND INTESTINAL FLORA IN DIABETES
Wu et al. (16) showed that metformin interacts with different gut bacteria, possibly through the regulation of metal homeostasis. Furthermore, microbiota-based interventions may reduce gastrointestinal symptoms associated with metformin administration, with a consequent improvement in medication compliance (40).
Probiotics exhibit metabolic benefits by improving the gut barrier and alleviating inflammation (41), which are also key to the development of ageing-related diseases (42). Health-associated Bifidobacterium spp. have been shown to be depleted with ageing but enriched in extremely aged healthy subjects (43). It has been proposed that the intake of probiotics might improve the integrity of intestinal epithelium and diminish the toll-like receptor-4 pathways to reduce pro-inflammatory signaling and to enhance insulin sensitivity (44,45).
PROBIOTICS AND INTESTINAL FLORA IN DIABETES
Fermented foods containing lactic acid bacteria, such as cultured milk products and yogurt, represent a source of ingestible microorganisms that may beneficially regulate intestinal health (46). They are thought to accomplish this through their effects on the existing gut microbiome, in addition to a possible induction of anti-inflammatory cytokines such as IL-10 (47). Based on these properties, foods enriched for these modulatory microorganisms are referred to as probiotics. Several groups have reported increased total bacterial loads after regular consumption of fermented milk or yogurt (48-51). Notable increases in beneficial gut bifidobacteria and/or lactobacilli have also consistently been observed with several different types of probiotics (52).
Individuals with metabolic disorders such as obesity and diabetes have been shown to have an intestinal imbalance when compared to healthy individuals (53,54).
THE MECHANISMS OF PROBIOTICS IN DIABETES
Some possible mechanisms behind the effect of probiotics on glycemic control are presented. Researchers have hypothesized several mechanisms of action according to the previous literature. The effect of probiotics in improving glycemic control can be firstly explained through the action of primary bile acids on the farnesoid-X-receptor (FXR). Certain strains of Lactobacillus and Bifidobacterium are known to possess the bile salt hydrolase enzyme (BSH). This enzyme can directly increase the levels of primary bile acid, which in turn binds and activates the FXR, leading to increased storage of glucose, decreased production of glucose from non-glucose nutrients, increased synthesis of insulin and increased secretion of insulin (55,56). Secondly, probiotics are also known to increase glucagon-like peptide (GLP) 1 and GLP 2, which are able to decrease low-grade inflammation associated with diabetes, and to decrease insulin resistance, which in turn decreases ß-cell toxicity and improves glycemic control. Furthermore, GLP-1 and GLP-2 also decrease hunger and increase satiety, thus decreasing energy intake, which collectively improve glycemic control (57). Another possible mechanism is the increased production of short-chain fatty acids (SCFAs) by mainly Bifidobacterium in the colon via its action on insoluble dietary fibers. These SCFAs, especially butyrate, can decrease insulin resistance by promoting pancreatic ß-cell differentiation, proliferation and development; increase secretion of GLP-1, thus increasing secretion of insulin, and decrease the release of pro-inflammatory cytokines by adipose tissue (58,59).
In conclusion, notwithstanding some limitations, these findings may have important implications for managing T2DM in patients by treating the microecological imbalance. These studies will also provide empirical evidence to address currently unresolved issues with the efficacy and safety of probiotics. Future research should focus on identifying the role of and the complex interaction among probiotics and the proportion of the various phyla in the gut of individuals.
CONCLUSIONS
All in all, probiotics have emerged as a possible therapeutic option, and prospective clinical trials have shown promising results in T2D patients. But up to now, there is still a dearth of such studies, so there was a need for high-quality studies to better understand the clinical effects of the intestinal flora on the occurrence and development of diabetes. This issue remains unanswered at present, primarily because large, long-term prospective trials of probiotic therapy are absent. However, several problems such as the possible side effects of long-term use of probiotics remain to be solved. The effects of probiotics are also slow, and some patients and doctors may be afraid of delaying illness.