INTRODUCTION
Dyslipidemia refers to the abnormal quality and quantity of lipid substances in plasma, the increase of cholesterol and/or triglyceride in plasma, and the decrease of high-density lipoprotein (1). Lipid exists in the form of binding lipoprotein in plasma, circulating and metabolizing through the intestinal tract and liver through exogenous/endogenous metabolic pathways, respectively, which is closely related to energy supply and atherosclerosis (2). At present, it is believed that a variety of acute and chronic diseases are closely related to dyslipidemia, such as acute pancreatitis, coronary heart disease, cerebral infarction, and fundus changes of hyperlipidemia (3-6). There is no name for dyslipidemia in TCM, but pathological products such as phlegm turbidity and blood stasis are quite similar to dyslipidemia, especially blood turbidity. For a variety of diseases, such as chest obstruction, heartache, syncope, stroke, etc., there is a syndrome of phlegm turbidity accumulation/blood stasis stagnation, and its pathogenicity is obvious (7,8).
At present, the drugs used in clinical intervention of dyslipidemia mainly include HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) (reductase inhibitors, phenoxy aromatic acids, nicotinic acid, cholic acid chelating agents, intestinal cholesterol absorption inhibitors, n-3 fatty acid preparations, etc. (9-13). The most widely used class is statins, which can reduce serum cholesterol and low-density lipoprotein, and also reduce triglycerides to a certain extent. However, some patients still have an intolerance to the above six lipid-lowering drugs, drug resistance, or poor compliance, which affect the prognosis of patients (14-17).
A Chinese herbal compound has great potential in the intervention of dyslipidemia. It has the characteristics of extensive effect, low toxicity and side effects, and stable curative effect (18). Its advantages in improving prognosis cannot be ignored. Bioinformatics is the result of integrating medicine, computer science, statistics, and other multidisciplinary theories and research methods, which can better analyze and predict the molecular mechanism of traditional Chinese medicine compound intervention in diseases, which is consistent with the multi-target, multi-channel, and multi-faceted treatment of traditional Chinese medicine (11-19,20). Therefore, bioinformatics can further effectively guide the connotation mining and new drug research and development of traditional Chinese medicine prescriptions to enrich the theory of traditional Chinese medicine and promote clinical development.
Based on this, this paper uses bioinformatic methods to study the potential molecular mechanism of the Xiexin capsule in the intervention of dyslipidemia from an overall perspective, taking the hospital preparation of the Xiexin capsule in the First Affiliated Hospital of Hunan University of Traditional Chinese Medicine as the starting point and provides some reference for subsequent research (Fig. 1).
METHODS
SELECTION OF EFFECTIVE COMPONENTS AND PREDICTION OF TARGETS OF THE XIEXIN CAPSULE
The Xiexin capsule is a hospital preparation of the First Affiliated Hospital of Hunan University of Traditional Chinese Medicine. It is composed of three herbs: Rheum officinale Baill, Scutellaria baicalensis Georgi, and Coptis chinensis Franch. The effective components and corresponding targets of the above three Chinese medicines were searched by the TCMSP database (https://tcmspw.com/tcmsp.php). Oral bioavailability (OB) and drug-likeness (DL) are two important indexes used to evaluate the gastrointestinal absorption of Chinese herbal medicines and the possibility of becoming potential drugs; OB refers to the speed and extent of the drug being absorbed into the human circulation, which describes the percentage of oral drug absorbed from the gastrointestinal tract and through the liver to the circulating blood. DL refers to the similarity between compounds and known drugs. Drug-like compounds are not drugs, but may become drugs. According to the official recommended screening criteria, OB ≥ 30 % and DL ≥ 0.18 were used as screening conditions (21,22). Use the UniProt database (https://www.uniprot.org/) to obtain the official target gene name.
THERAPEUTIC TARGETS FOR DYSLIPIDEMIA
Using “hyperlipidemia”, “hypertriglyceridemia” and “hypercholesterolemia” as keywords, the Genecards (https://www.genecards.org/), OMIM (https://omim.org/), PharmGKB (https://www.pharmgkb.org/), TTD (http://db.idrblab.net/ttd/), and DrugBank (https://go.drugbank.com/) disease databases were searched to obtain the disease targets of dyslipidemia. DrugBank was first searched for indications and the corresponding disease targets of drugs were obtained according to the search results. The disease targets obtained in Genecards were filtered according to a relevance score ≥ 1.
CONSTRUCTION OF COMPONENT-TARGET MAP
To understand the interaction between the predicted targets of the main active components of the Xiexin capsule and the targets of dyslipidemia, the predicted targets of the Xiexin capsule and the related targets of dyslipidemia were crossed using the Perl software to obtain the key genes of drug-disease and the intersection of drug targets and disease genes. The drug target-disease intersection was aggregated into a file and imported into Cytoscape 3.8.0 software to construct the component-target network diagram by using its Network Analyzer function.
PPI NETWORK CONSTRUCTION AND TOPOLOGY ANALYSIS
The obtained intersection target genes were imported into the String platform (https://string-db.org/), species was set as 'Homo sapiens', the combined score (the lowest interaction score) was set to 0.4, and the free nodes were removed to obtain the protein interaction network diagram. Thus we can get the core genes of the Xiexin capsule in the treatment of dyslipidemia. The Cytoscape 3.8.0 software was used to download CytoNCA, and further topology analyses were carried out according to network centrality.
GO AND KEGG ENRICHMENT ANALYSIS
DOSE, cluster profiler, enrichment plot, path view, and other related packages of R language were used to perform gene ontology (GO) function enrichment analysis and Kyoto gene and genome encyclopedia (KEGG) pathway enrichment analysis on the overlapping genes. GO function enrichment analysis described the possible forms of molecular functions of gene products, involved biological processes, and the cellular environment. KEGG pathway enrichment analysis refers to the most significant biological process obtained by classifying known genome annotation information. It can predict the potential mechanism of the Xiexin capsule active ingredients in the intervention of dyslipidemia. The first 10 items of significantly enriched BP (biological process), CC (cell component), and MF (molecular function) were selected to draw the histogram. The first 30 pathways with significant enrichment were selected to draw the bubble diagram.
MOLECULAR DOCKING
Select the top 3 active compounds in drug-component-target as small molecule ligands: quercetin, wogonin, baicalein. Molecular docking was performed with the first three target proteins of the six key targets obtained after PPI network topology analysis, namely ESR1 (estrogen receptor 1), MAPK14, and HSP90AA1. The SDF file of the 2D structure of the active ingredient was downloaded from the PubChem CID database (https://pubchem.ncbi.nlm.nih.gov), and the 2D structure of the core gene-related proteins was downloaded from the PDB database (http://www1.rcsb.org/). The ligands and non-protein molecules(such as water molecules) in the target proteins were removed by PyMOL software and then saved as the PDB format file. AutoDock Tools 1.5.6 software was used to hydrogenate the crystal structure and calculate the charge of the protein. All ligand and receptor files were stored in PDB format. The Autodock Vina software was used for molecular docking, and the lowest binding energy was calculated. The PyMol software was used to generate a molecular docking model diagram.
RESULTS
SELECTION OF EFFECTIVE COMPONENTS AND PREDICTION OF TARGETS OF THE XIEXIN CAPSULE
In the TCMSP database, 66 compounds were screened under the conditions of OB ≥ 30 % and DL ≥ 0.18, including 16 Rheum officinale Baill, 36 Scutellaria baicalensis Georgi, and 14 Coptis chinensis Franch. Uniprot database was used to standardize the corresponding targets of effective components, and ' Reviewed ' and ' Human ' were selected to download the standard target name. By matching and deleting duplicate values, 114 targets were finally obtained.
THERAPEUTIC TARGETS FOR DYSLIPIDEMIA
Genecards, OMIM, PharmGkb, TTD, and Drugbank disease databases were, and the number of dyslipidemia targets was, 897, 13, 580, 16, and 68 after screening and removing the repeated values. The intersection of drug targets and disease targets was 87 (Fig. 2).
CONSTRUCTION OF COMPONENT-TARGET MAP
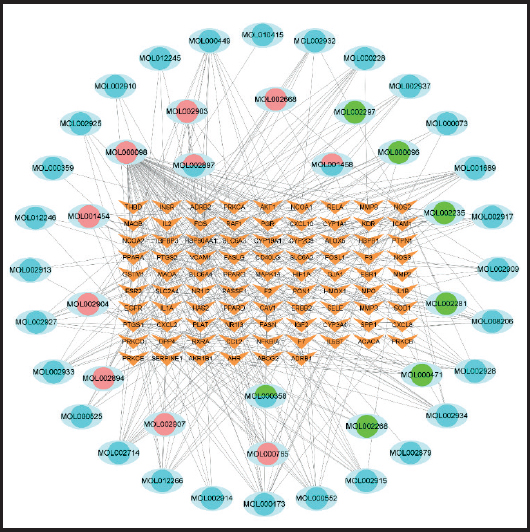
The intersection of predicted targets of the Xiexin capsule and related targets of dyslipidemia was imported into the Cytoscape 3.8.0 software, and its Network Analyzer function was used to construct a component-target network diagram. We adjusted area size, color depth, and sorting of nodes (target genes) in the network to some degree. The combined score is represented by the size of the line, and the drug-component-disease target diagram was finally obtained (Fig. 3). A total of 132 nodes, 384 edges was obtained. It can be seen from the figure that the active ingredients of MOL000098 contain the most relevant target genes (B67), and the active ingredients MOL000173, MOL002714, MOL000449, and MOL001689 are related to 17, 15, 13, and 12 targets, respectively. Among them, PTGS2 is the largest number of effective components corresponding to target genes(40), followed by PTGS1 (36), HSP90AA1 (35), NCOA2 (nuclear receptor coactivator 2) (28), and NOS2 (23).
PPI NETWORK CONSTRUCTION AND TOPOLOGY ANALYSIS
The selected Xiexin capsule and 87 common targets of dyslipidemia were submitted to the STRING platform. The species first located 'Homo sapiens', the combined score (the lowest interactive score) was set to 0.4, and the free nodes were removed to obtain the PPI network of the Xiexin capsule. The reader may visualize the files generated by the STRING database through Cytoscape 3.8.0 (Fig. 4). The network involves 23 nodes and 63 edges, in which the nodes represent the target gene and the edges represent the interaction between the target genes. CytoNCA of Cytoscape 3.8.0 was used for topological analysis, and relevant indicators were selected: betweenness centrality (BC), closeness centrality (CC), degree centrality (DC), eigenvector centrality (EC), local average connectivity (LAC), and network centrality (NC). Gene-related indicators were screened, and genes greater than the median of all indicators (BC > 8.63, CC > 0.5, DC > 6, EC > 0.21, LAC > 2.3, NC > 3.6) were selected for subsequent analysis. After one screening, six core genes were finally obtained and sorted according to DC. The core genes were ESR1, MAPK14, HSP90AA1, PTGS2, NCOA2, and NCOA1 (nuclear receptor coactivator 1) (Fig. 5).
GO AND KEGG ENRICHMENT ANALYSIS
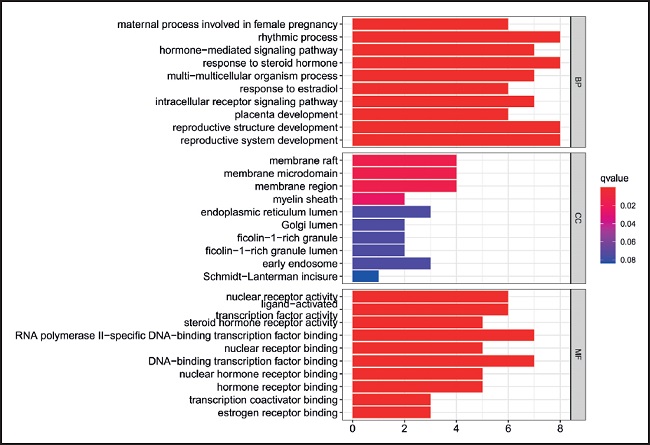
DOSE, cluster profiler, enrichment plot, path view, ggplot2, and other related programs of R language were used to conduct gene enrichment analysis on 87 potential targets of Xiexin capsules, and 969 GO enrichment entries were obtained, including 867 biological processes (BP), 4 cellular components (CC), and 98 molecular functions (MF). The top 10 GO entries of BP, CC, and MF were selected to draw the histogram, and the top 30 entries of KEGG were selected to draw the bubble diagram (Fig. 6 and Fig. 7). Bioprocess enrichment showed that hormone-mediated signaling pathways, steroid hormone response, lipid transport and metabolism, cholesterol storage regulation, cyclooxygenase pathway, and other biological processes played an important role in the intervention with the Xiexin capsule in dyslipidemia. KEGG enrichment analysis showed that the main pathways related to dyslipidemia were the PPAR signaling pathway, IL-17 signaling pathway, PI3K-Akt signaling pathway, and FcεRI signaling pathway (Fig. 8 and Fig. 9).

Figure 8. KEGG enrichment results and potential regulatory network prediction of Xiexin capsules: the PPAR signaling pathway
MOLECULAR DOCKING
The first three main active compounds in the “Drug-Component-Target” network with the closest interaction with the target were selected as small molecule ligands: MOL000098 (quercetin), MOL000173 (wogonin), and MOL002714 (baicalein). Molecular docking was performed with the first three core target proteins obtained after PPI network topology analysis, namely ESR1, MAPK14, and HSP90AA1. Molecular docking was performed using the Autodock Vina software and the minimum binding energies of each protein to small molecular ligands were calculated (Table I). The smaller the binding energy, the greater the affinity between the receptor and the ligand, and the more stable the spatial conformation. We used the PyMol software for visualization (Fig. 10). The results showed that the binding energy of each compound to protein was less than -4.0 kcal·mol-1, indicating that each compound could bind to protein closely. Baicalein had the highest affinity with ESR1, wogonin had the highest affinity with MAPK14, quercetin had the highest affinity with HSP90AA1.
DISCUSSION
MODERN SCIENTIFIC CONNOTATION OF COMPATIBILITY OF THE XIEXIN CAPSULE
The Xiexin capsule is a hospital preparation developed by Professor Chen Xinyu in the First Affiliated Hospital of Hunan University of Traditional Chinese Medicine, which has been used safely for more than 10 years. The main components are Rheum officinale Baill, Coptis chinensis Franch, and Scutellaria baicalensis Georgi. The traditional Chinese medicine compound was born in Zhongjing Xiexin Decoction (Synopsis of Golden Chamber) cloud: lack of heart qi, vomiting, bleeding, Xiexin Decoction. Professor Chen Xinyu believes that dyslipidemia is closely related to the evil of blood turbidity. Blood turbidity can be caused by an imbalance of cooling and heat in the blood, or by cold coagulation, or heat accumulation. Blood turbidity is deposited in the wall of the pulse, blocking the wall of the pulse, and qi and blood stasis. It can lead to phlegm turbidity, blood stasis, daily heat, or cold knots, circulation, and malignant causality. Therefore, Professor Chen Xinyu from the “fire evil” point of view, “Qingfa” argument, uses Xiexin capsule to reduce fire evil and clear blood turbidity. This study found that the main key active ingredients in the Xiexin capsule were quercetin, wogonin, baicalein, stigmasterol, acacetin, etc. Through the synergistic effect of the traditional Chinese medicine compound, it may activate the PPAR signaling pathway, the IL-17 signaling pathway, PI3K-Akt signaling pathway, FcεRI signaling pathway, and then regulate downstream related targets to intervene in dyslipidemia, which is in line with the principle of syndrome differentiation and treatment of traditional Chinese medicine. Huanglian is the monarch in the prescription, purging the heart and solid fire, treating the root of the disease, eliminating evil fire is turbid and clear. Modern pharmacology shows that berberine in Coptis chinensis Franch can reduce the levels of serum total cholesterol, triglyceride, and low-density lipoprotein in animal models. The mechanism is to activate the PPAR signaling pathway and regulate the mRNA and protein expression of low-density lipoprotein receptor (LDLR) and cholesterol 7α-hydroxylase (CYP7A1 [cytochrome P450 family 7 subfamily A member 1]) (23-25). Scutellaria, Rheum officinale Baill as the subject medicine, scutellaria' bitter to dry wet, cold to win the heat, with Coptis heart and purging heart fire. Badawy found that the levels of oxidative stress, apoptosis, and inflammatory response in cisplatin-induced nephrotoxicity rats were decreased by using wogonin, and the inflammatory response in the process of renal damage was well inhibited (26). By activating the PPAR signaling pathway and IL-17 signaling pathway, the downstream PPAR-γ was up-regulated, while the activities of IL-1β, TNF-α (tumour necrosis factor α), NF-kB (nuclear factor kappa B), and caspase-3 were inhibited to varying degrees (27). Li further confirmed that wogonin can increase the expression of PPAR-γ, inhibit the expression of TNF-α and IL-6, and also has a significant anti-inflammatory effect in alcoholic liver disease animal models. Rheum officinale Baill bitter cold, good at heat down, auxiliary Huanglian to dissipate the heat of the heart and diaphragm, and blood stasis, disease care, clear blood turbidity to eliminate chronic illness (28). Through the analysis of Rheum officinale Baill extract, it is found that the stilbene compound deoxy radish sulfur contained in Rheum officinale Baill extract can significantly reduce the expression of inflammatory/apoptosis-related proteins such as NF-κB, PTGS2, and iNOS, and its role involves a series of signaling pathways such as PI3K-Akt/MAPK/IL-17 (29). An animal study showed that the Xiexin Decoction increased the mRNA levels and activities of key enzymes for short-chain fatty acid synthesis such as acetate kinase (ACK) and methylmalonyl COA decarboxylase (MMD), thereby increasing the production of short-chain fatty acids by intestinal microflora, further improving insulin sensitivity and reducing fat accumulation (30).
REGULATORY NETWORK OF THE XIEXIN CAPSULE ON DYSLIPIDEMIA
Based on the previous studies of the research group, the Xiexin capsule has a significant effect on the intervention of dyslipidemia. However, its potential molecular mechanism is not clear. The results showed that the active components of Xiexin capsule may regulate ESR1, MAPK14, HSP90AA1, PTGS2, NCOA2, NCOA1, and other targets by activating/inhibiting the PPAR signaling pathway, IL-17 signaling pathway, PI3K-Akt signaling pathway, and FcεRI signaling pathway, and then play an intervention role in dyslipidemia. Based on the above studies, it is speculated that the Xiexin capsule may intervene in dyslipidemia through two aspects. First, it directly regulates the related pathways of lipid metabolism disorders, such as the PPAR signaling pathway, and mediates downstream ApoA, CETP, PLTP, MTP, and other proteins, as well as LDLR, LRP/LCAT, and other receptors and enzymes, thereby directly regulating the generation and metabolism of lipids. On the other hand, it may activate/inhibit oxidative stress, inflammatory response, and apoptosis-related pathways such as IL-17 signaling pathway, PI3K-Akt signaling pathway, and FcεRI signaling pathway by participating in the oxidative and inflammatory reactions of cells in the body, and further, affect the downstream Bcl-2/Caspase9/p53/NF-κB/PTGS2/iNOS/ERK and other related proteins. After the formation of lipid accumulation, it can reduce the level of inflammatory infiltration in the blood vessel, delay the formation and development of atherosclerotic plaques, and reduce cell apoptosis, and ultimately prevent and treat atherosclerosis. Through literature review, it is found that peroxisome proliferators-activate receptors (PPAR) are nuclear hormone receptors activated by fatty acids and their derivatives. The essence of PPAR is a ligand-dependent transcriptional regulator that regulates intracellular lipid metabolism, including three subtypes: PPARα, PPARβ/δ, PPARγ (31,32). PPARα mainly regulates lipid metabolism in the liver and skeletal muscle, then affecting clearing circulation or intracellular lipids. PPARβ/δ regulates lipid oxidation, and PPARγ is involved in adipocyte differentiation and sugar uptake (33,34). In the early studies, by comparing the gene array analysis of high-fat diet and normal diet animals, it was found that the serum cholesterol of animal models with a long-term high-fat diet increased, and the PPAR signaling pathway was activated. The downstream gene expressions of LPL (lipoprotein lipase), PPARα, PPARγ, CD36, AQP7 (aquaporin 7), CPT1b (carnitine palmitoyltransferase 1B), and FABP2 (fatty acid binding protein 2) were significantly up-regulated (35,36). The expression of PPARα, CYP7A1, APOB, APOE, and HMGCS1 mRNA was increased in the animal model by drug intervention, which indicated that the PPAR signaling pathway was activated in the process of lipid metabolism, and the downstream genes of PPARα, CYP7A1, APOB, APOE, and HMGCS1 were regulated, which promoted the conversion of cholesterol to bile acid, and reduced the level of cholesterol in the serum by increasing the excretion of bile acid (37). In the process of clinical diagnosis and treatment, the control of blood lipids is not the ultimate goal, and the prevention and treatment of long-term complications such as atherosclerosis, non-alcoholic liver disease, cerebrovascular disease, and kidney disease are more valuable (38). IL-17, PI3K-Ak, FcεRI, and other signaling pathways are involved in the occurrence and development of several complications of dyslipidemia in terms of inflammatory response and apoptosis. Recent studies have shown that the above pathways may improve lipid clearance and metabolism by reducing the inflammatory response and adjusting intestinal flora (39-42). A drug study on atorvastatin found that the levels of blood lipid-related proteins, including TC (total cholesterol) (t9, LDL (low density lipoprotein), HDL (high-density lipoprotein), and TG, were improved to varying degrees by using lipid-lowering drugs to intervene in patients with hyperlipidemia. Accordingly, the downstream genes of the IL-17 pathway, such as CRP (C-reactive protein), MCP-1 (macrophage chemoattractant protein 1), IL-1β, IL-6, and TNF-α, are reduced, suggesting that the regulation of dyslipidemia may require the mediation of anti-inflammatory pathways (43).
CONCLUSION
Based on the results of this study and the review of the relevant literature, it is speculated that Xiexin plays a role against dyslipidemia through two mechanisms. On the one hand, the PPAR signaling pathway is activated to regulate downstream targets such as ApoA, CETP, PLTP, MTP, CYP7A1, and peroxisomal acyl-coenzyme A oxidase (ACOX), thus directly participating in the process of lipid-binding/transport/metabolism. On the other hand, by activating/inhibiting the IL-17 signaling pathway, PI3K-Akt signaling pathway, and FcεRI signaling pathway, the downstream targets of ESR1, MAPK14, HSP90AA1, PTGS2, NCOA2, NCOA1, CRP, MCP-1, IL-1β, IL-6, and TNF-α are regulated, and then intervene to improve inflammatory response, reduce apoptosis and regulate the intestinal flora, thereby indirectly interfering with dyslipidemia and preventing long-term target organ complications (Fig. 11).