INTRODUCTION
Obesity is a condition characterized by an abnormal increase in adiposity that promotes the development of metabolic dysfunction (1). However, exercise counters this phenomenon in different ways. One of them is to promote the ability of the adipocyte to produce heat (thermogenesis) (2,3). Here, the adipocytes combust nutrients in a “futile” manner in the mitochondria, as a measure to counter energy excess by releasing it as heat, a phenomenon that occurs by the proton flux in the mitochondria, facilitated by uncoupling proteins (uCP), as uCP1 (4). This phenomenon was firstly reported in brown adipocytes; however, in recent years, it has been described in white adipose tissue under the name of “browning” or “beiging” (5).
Considering the potential metabolic benefits of adipocyte browning, irisin has been proposed as a key protein, given its ability to stimulate transcriptional and translational changes towards this process (6). Interestingly, the level of circulating irisin is higher than insulin and lower than leptin, with a molecular weight of 12 kDa (7). Briefly, it was initially described in 2012 by Boström et al. where irisin increases in human and mouse plasma (~2-fold from baseline) were seen after 3 and 10 weeks of exercise, respectively, changes that were accompanied by mRNA increases of FNDC5 (membrane-bound irisin uncleaved form) in mice skeletal muscle (8). Indeed, the C-terminal tail of FNDC5 is in the cytoplasm, whereas the extracellular N-terminal part is released into the circulation as irisin (9). From there, the mechanisms by which irisin acts are still elusive; however, the induction of proteins responsible for browning of white adipose tissue (e.g., cell death inducing DFFA like effector A [Cidea], uncoupling protein 1 [ucp1], PR/SET domain 16 [Prdm16], peroxisome proliferator-activated receptor gamma coactivator 1-alpha [Pgc1a]) by the presence of irisin is one of the most investigated pathways to date (3,10). Thus, white adipose browning has been suggested as a metabolic benefit derived from exercise, where irisin seems to be a key mediator of that response.
However, most of the studies in the field have been conducted in cell and/or animal models, where most of the clinical data available were obtained in healthy individuals (11,12). While animal studies are congruent with regard to the relationship between physical exertion and irisin release, the RESULTS from human studies are less than clear. Moreover, in the context of obesity and insulin resistance, the evidence is conflicting. For instance, Batitucci et al. described ~3-fold increases in circulating irisin levels in women with obesity after 10 weeks of aerobic exercise (13); however, others with a similar exercise program failed to see significant irisin changes in women with overweight or obesity (14).
Because of the discrepancy seen in clinical studies regarding the effects of exercise on irisin in the context of overweight and obesity, our question is: in adults with overweight and obesity, what are the effects of exercise on irisin levels in clinical studies? Therefore, this review aims to systematically search and analyse the literature available in this topic, to potentially understand the sources of this discrepancy and highlight the gaps in the field.
METHODS
Three databases were used to conduct the literature searches: PubMed, ScienceDirect, and Medline. Original articles that included human participants published between 2010 and August 2021, written in English, were considered. Selected studies included participants between the ages of 18 and 59, with overweight, obesity, impaired glucose tolerance, and/or type 2 diabetes. Studies that included subjects with associated conditions such as, cancer, heart failure, or stroke and studies with incomplete data were excluded.
The search and selection of studies were conducted as follows: two authors (CB-R and AL-T) reviewed the results from the three databases and selected the studies of interest by reading: a) title; b) abstract; and c) main text. In case of any disagreement between them, a third author (SM-H) decided in favour/against the inclusion of a particular study.
Searches strategies used were as follows: PubMed: “Exercise” [Mesh] AND “FNDC5 protein, human” [Supplementary Concept] AND “Overweight” [Mesh]; Science Direct: (Exercise OR physical activity OR aerobic exercise) AND (Irisin OR FNDC5) AND (overweight OR Obesity); MEDLINE: (MH “Exercise+”) AND “Irisin” AND (MH “Overweight+”). As the primary outcome, we considered irisin changes (circulatory and/or from tissues) by exercise. For this, we defined as baseline (1.0-fold-change) the pre-exercise irisin levels of the participants of each study. Therefore, increases and decreases were expressed as higher or lower than 1 respectively.
RESULTS
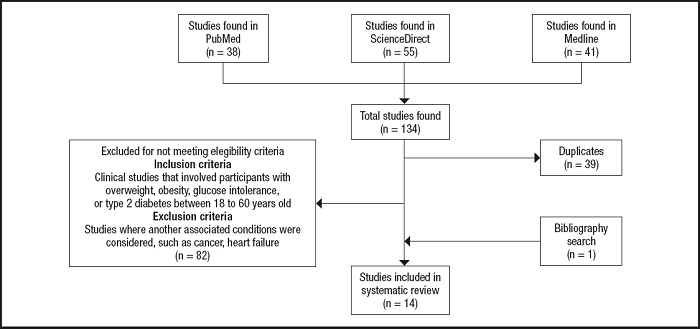
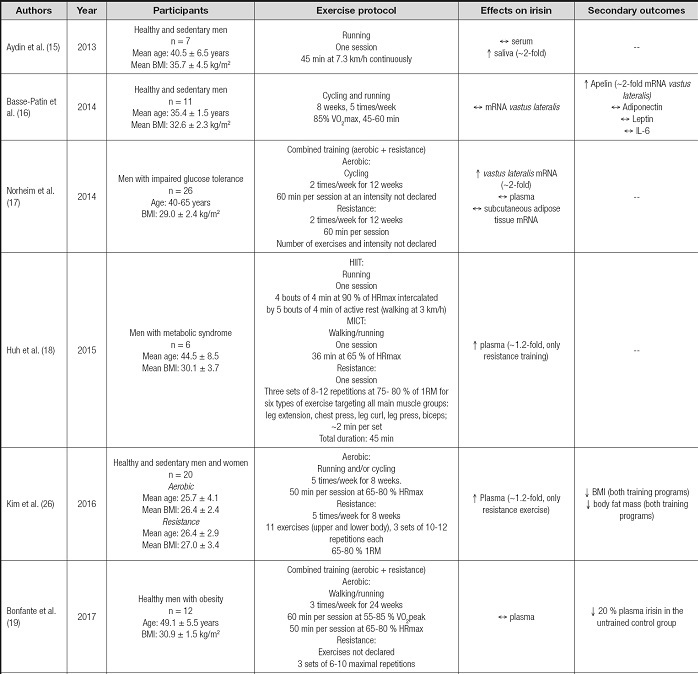
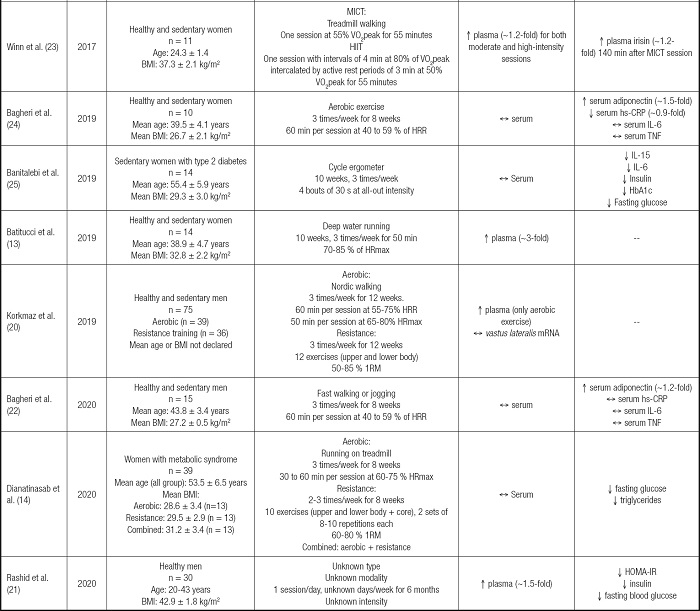
From 134 articles found, 14 studies were included in this review as described in figure 1. From these, 8 included only men as participants (15-22), 5 studies focused on women (13,14,23-25) and only one included both men and women (26). In terms of exercise regimes used, the most frequent was an aerobic exercise with 11 studies (13-16,18,20,22-26), followed by resistance (14,17,18,20,26) and combined exercise training programs (17,19). One study did not describe the exercise prescription used (21). Eleven studies considered exercise training (≥ 8 weeks) (13,14,16,17,19-22,24-26), whereas three analyzed the acute effects of exercise after a single session (15,18,23). Among the studies that analyzed the chronic effects of exercise, the frequency ranged from one session to 5 sessions/week, with a duration of 45-60 minutes per session, where the exercise programs lasted from 2 to 6 months.
Irisin measurements were made from serum/plasma samples in 13 studies (13-15,17-26), from which only 6 reported significant increases after exercise (13,18,20,21,23,26), changes that ranged from 20 to 300 % compared to pre-exercise levels. In addition, three studies measured the vastus lateralis irisin mRNA levels after exercise training (16,17,20), and only 1 reported a significant increase (~2-fold) (17). Also, irisin was measured from subcutaneous adipose tissue (17) and saliva samples (15), where a ~2-fold increase in its protein levels was found in the latter.
A summary of the studies selected for this review is listed in table I.
Table I. (cont.). Irisin changes in selected studies.
BMI: body mass index; VO2max: maximum oxygen consumption; mRNA: messenger ribonucleic acid; IL-6: interleukin 6; HIIT: high-intensity interval training; MICT: moderate-intensity constant training; HRmax; maximum heart rate; 1RM: maximum one-repetition; VO2peak: peal oxygen consumption; HRR: heart rate reserve; hs-CRP: highly sensitive C reactive protein; TNF: tumor necrosis factor; IL-15: interleukin 15; HbA1c: glycated hemoglobin; HOMA-IR: homeostatic model assessment-insulin resistance.
DISCUSSION
This report aimed to systematically review the literature available regarding the effects of exercise on irisin levels in clinical studies involving subjects with overweight or obesity. As main findings, most of the studies measured irisin exclusively in the circulation, reporting conflicting RESULTS, considering that less than half of the articles reviewed reported significant changes (increases) in irisin concentrations. Moreover, irisin levels in specific tissues (i.e., skeletal muscle and white adipose tissue) were scarcely measured, and only in terms of transcriptional levels.
The variability in terms of irisin changes after exercise is concerning when trying to establish this response as a metabolic benefit derived from this intervention. Interestingly, a handful of studies have attempted to elucidate if the exercise type/modality influences irisin response. For instance, Blizzard LeBlanc et al. compared the effects of aerobic and resistance training effects on the irisin levels of youth with obesity. After one session of each type of exercise, they found that only aerobic training induced a significant irisin increase (27). In opposition, Huh et al. compared two types of aerobic exercise and resistance training on the irisin levels of adult men with metabolic syndrome, finding that resistance but not aerobic training increased irisin plasma levels by 20 % (18), increases of a similar magnitude as described in other study involving young adults with overweight after 8 weeks of resistance training (26). These results suggest that age might play a role considering that most of irisin comes from skeletal muscle and white adipose tissue, organs that, in the case of youth/young adults, could be in a better metabolic state than with adults (28).
In line with this, a systematic review by Fox et al. described that fitness level was a strong predictor of exercise-derived increases of irisin in the circulation, where this change was nearly two-fold compared to unfit counterparts (29). Complementarily, another systematic review with meta-analysis found that only long-term resistance training, not aerobic exercise, significantly increased irisin levels in adults (30), which could indicate that a more focused intervention on skeletal muscles might be a more potent stimulus to induce irisin. It is worth mentioning that the authors of this review included studies involving lean subjects with moderate physical activity levels. Moreover, the acute vs. chronic influence of exercise on irisin could also be a source of diversity. For instance, Winn et al. described plasma irisin increases after single aerobic exercise sessions in women with overweight (23); however, these changes were not seen after 8 (24) or 10 weeks (25) of aerobic exercise. Therefore, when analysing irisin responses, differentiation should be made between acute or chronic adaptations to exercise. In addition, confounding factors at sample extraction might also be contributing to the variability seen here. In a study included here, authors measured irisin from saliva samples after exercise or hot-water baths (Turkish baths), finding a similar 2-fold irisin increase (15). Even when interesting, measuring protein levels from saliva after exercise is challenging given the dehydration involved a factor that per se induces changes in saliva's protein concentration (31), affecting the results' interpretation. Therefore, considering the several confounding factors that might influence irisin levels during obesity and exercise, it is recommended the inclusion of multivariate analysis as statistical tools when analysing the irisin response to exercise, to reach clearer conclusions.
Considering that the main sources and targets of irisin are insulin-sensitive tissues (e.g., skeletal muscle, white adipose tissue, and liver), it is concerning that only a handful of studies investigates irisin changes directly in tissues during exercise (16,17,20). This overreliance on circulating measurements could originate in unprecise conclusions. For instance, Archundia-Herrera et al., in adolescent women, investigated the acute effect of two types of aerobic exercise (MICT and HIIT) in vastus lateralis and plasma irisin protein level. Even when no changes were detected in plasma, a ~2-fold increase was seen only after HIIT in the skeletal muscle (32). This could indicate that there might be latency between irisin induction derived from exercise and its translation into a higher circulatory availability. For this purpose, studies such as the one conducted by Winn et al. are useful to track irisin changes after one exercise session. They reported that ~2 hours after moderate-intensity exercise, irisin levels were significantly higher than pre-exercise values; however, after a high-intensity interval training session, these increases were seen only during the exercise session (23). These findings suggest that this potential latency between tissue and circulatory levels of irisin could also depend on the intensity of exercise. Therefore, these factors altogether might help to explain the variability found in the circulatory levels of irisin after exercise.
This review has several limitations to be considered. First, even when studies with children, adolescents, and older adults were not included, the broad age range of the participants included in this review could negatively impact the comparability of the studies presented here, considering the significant impact of age on systemic metabolism. Secondly, the sample sizes of the studies reviewed here were usually low (8 out of 14 studies had less than 15 participants), which in conjunction with the high variability of irisin concentrations, particularly in the circulation, could hinder the possibility to reach more robust conclusions. Moreover, the high variability of exercise prescription, even when they were under the same type (aerobic or resistance training), contributes to the scarce agreement between the studies' outcomes.
In conclusion, the literature available suggests that exercise induces increases in the circulatory concentrations of irisin in subjects with overweight or obesity. However, this response is highly variable and is not commonly found throughout the studies included in this review. This variability could be influenced by several factors, such as age, fitness level, exercise type, the sample used (serum, plasma, saliva, or tissue), and sample extraction time frame (Fig. 2). In addition, most of the clinical studies in the field haven't investigated how exercise modifies irisin expression in its known tissue sources, such as skeletal muscle and adipose tissue, where it might be possible that different types of exercise could affect FNDC5 cleavage rates differently, particularly in skeletal muscle. Therefore, a more integrative approach is needed in this field to have a clearer understanding of this phenomenon.
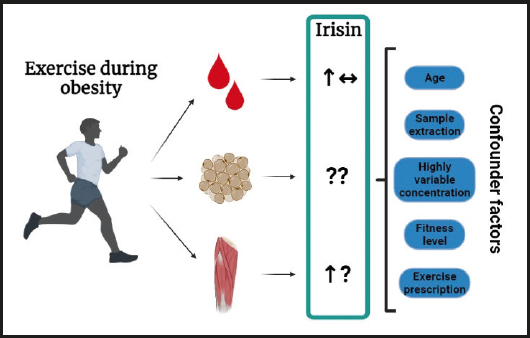
Figure 2. Summary of the effects of exercise on irisin during obesity. Exercise might induce increases in circulatory levels of irisin, however, several studies failed to report any changes whatsoever. In skeletal muscle, incipient evidence suggests that exercise increases transcriptional levels of irisin, however, no studies were found in white adipose tissue in humans with overweight/obesity. Several confounder factors were highlighted among the studies, which could explain the variability of the results presented here (↑: increase; ↓: decrease; ↔: no change; ?: unclear/unknown).