INTRODUCTION
Medical nutrition therapy (MNT) is a commonly neglected part of diabetes management despite the fact of being cited virtually in all professional guidelines as an essential item for proper glycemic control (1-5). Instead of adding another drug with its inherent side effects, especially hypoglycemia, a proper nutrition based glycemic control is a good option in patients slightly above A1C target levels (between 6.5 and 8.5 %). One of the major obstacles for MNT routine implementation is the complexity of the prescribed meal plans (6). Lack of time is also pointed out by patients as another issue. In order to tackle these unmet needs, there are meal replacements specifically designed for people with diabetes, which helps to control eminently postprandial glycemia (PPG) (7).
There is a well-defined relationship between PPG and increased incidence of acute myocardial infarction and all-cause mortality in patients with type-2 diabetes, regardless of A1C values (8-10). Among the meals, breakfast is the one with the greatest impact in PPG. This contribution is greater in patients with reasonable control: the lower the A1C, the greater the impact of GPP on total glycemic control (11). Therefore, PPG of the breakfast seems a fast endpoint to be achieve in an pivot clinical trial.
The PPG can be accessed using intermittent scanning continuous glucose monitoring system (isCGM). The isCGM measure continuously the interstitial glucose, which have a good correlation with plasmatic glucose. Therefore, the incremental area under the curve (iAUC) can be calculated, and it is considered a good parameter to compare different interventions regarding PPG (12,13).
Nutren Control® is a recently developed glycemia targeted specialized supplement (GTSS). This new GTSS was selected because it was designed to replace the breakfast for a patient with diabetes, including an adequate amount of calories - one portion of 200 ml provides 208 kcal, and a well balanced composition of macronutrients - one portion has 28 % of carbohydrates, 29 % of protein and 43 % of lipids. Comparing to a usual breakfast, there is a reduction of carbohydrates and a relative increase in proteins and lipids, modifications that Pedersen E et al demonstrated the effectiveness in reducing PPG after breakfast in patients with T2D (13).
This is the first study to address this new GTSS metabolic efficacy in T2D patients. CONTROL DIABETES study was a randomized crossover, single center, clinical trial that compared Nutren Control® as a breakfast substitute to a standardized diet. The CONTROL DIABETES study has the objective to focus on PPG after breakfast.
MATERIAL AND METHODS
STUDY POPULATION
The study was performed at the Instituto Brasil de Pesquisa Clínica (IBPClin), a dedicated clinical trial research center located in Rio de Janeiro, Brazil.
Subjects with T2D and suboptimal control in monotherapy with metformin were invited to participate, during the first semester of 2021. The individuals were invited to participate in the study by searching the IBPClin database. Participants were included if they had T2D defined by: fasting plasma glucose (FG) ≥ 126 mg/dL in two different occasions, glucose ≥ 200 mg/dL after oral glucose tolerance test (OGTT), random plasma glucose test ≥ 200 mg/dL, A1C test ≥ 6.5 % or in use of any dosage of metformin. Participant ages were between 30 and 70 years and the A1C test should have been between 6.5 and 8.5 %. Metformin should have been in a stable dose for at least 2 months before enrolment. Participants were excluded if they had type 1 diabetes, body mass index (BMI) ≥ 35 kg/m2, previous bariatric surgery, chronic diarrhea or disabsorptive disease, current use of medications that affect blood glucose (such as corticosteroids, cyclosporine, interferon), use of any glucose-lowering agent other than metformin within 3 months before screening, current illicit drug use, current alcoholism, pregnant or lactating women.
EXPERIMENTAL PROTOCOL
The CONTROL DIABETES study was an intervention, prospective, randomized, controlled, crossover trial to compare the effect of a new GTSS and a standardized breakfast on postprandial glycemia (PPG). The isCGM was used to determine the primary endpoint: the difference between the interventions regarding the incremental area under the curve (iAUC) of the PPG (3 hours after intervention); and to determine the secondary endpoints: difference between the interventions regarding the glycemic peak, postprandial glucose excursion (PPGE), mean blood glucose (MBG) and time in range (TIR).
The study was approved by the Ethics Committee of the Procardíaco Hospital (PROCEP), registered as CAAE 39228120.8.0000.5533, and received the number 4.354.456. All participants were admitted only after providing their informed consent. The study was carried out according to principles of the Declaration of Helsinki and also in abidance with the International Conference on Harmonization (ICH)/Good Clinical Practice (GCP) guidelines.
Participants who were eligible were randomized in two groups, which differ from each other regarding the sequence of intervention. Group A received the GTSS first for 7 days, followed by standardized breakfast also for 7 days; Group B received standardized breakfast first for 7 days, followed by the GTSS for 7 days. There was no washout period between the interventions, since it was not expected that interventions would influence the glycemia in the following day after the discontinuation. Total duration of intervention for each participant was 14 days (7 days of GTSS and 7 days for standardized breakfast) (Fig. 1).

Figure 1. Experimental protocol. Screening was performed in visit V-1. Participants who are eligible were randomized to group A or group B, which differ from each other regarding the sequence of intervention, at treatment visits V0 and V1. At visit V1 and V2 data from isCGM were collected after treatment intervention. V3 was performed to check safety.
Randomization follows the setting of 36 random numbers, ranging from 1 to 2, generated in the site Research Randomizer (https://www.randomizer.org) by one consultant outside of IBPCLIN. Only one person at IBPCLIN, responsible for the regulatory process, knew the randomization list; this person did not have direct contact with the researcher. The study coordinator, that was “blind” and had no access to the randomization list, assigned to interventions the participants.
INTERVENTIONS
Each package of the GTSS, named Nutren Control®, has 200 ml, considered as one portion. GTSS composition is described in table I.
The standardized breakfast was created by a nutritionist specialized in diabetes, following the recommendation of American Diabetes Association and other professional diabetes societies (1-5), and it is described in table II. It was isoenergetic compared to the GTSS (237 vs 208 kcal, respectively), differing regarding macronutrient composition (Table III).
Table III. Comparison between the composition of Nutren Control® and the standardized breakfast.
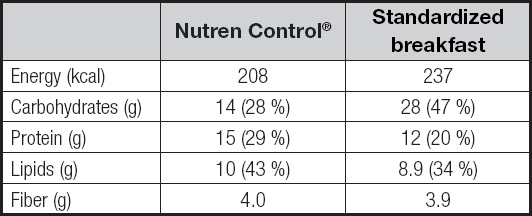
g: grams; kcal: kilocalories.
The research center provided the participants the standardized breakfast, divided in 7 bags, as well as the GTSS (7 bottles with 200 ml of the GTSS). Individual meals were packed in bags, clearly marked with the meal's date and time. They were asked to eat all, and only, the food that was provided. In the same way, they were asked to consume the entire content of the GTSS bottle.
Participants were oriented to use the intervention once a day, during breakfast time, for 7 consecutive days. They were asked to have a standard dinner with 500 kcal and 40 g of carbohydrate every day in the nights before the interventions, and to fast for 8 hours before commencing the interventions. After breakfast, they were asked not to consume any meals for the next 4 h. Moreover, participants were also asked to maintain similar, habitual physical activity for both interventions. Participants were not allowed to intake any alcohol during the trial period.
MEASUREMENTS OF BLOOD GLUCOSE PROFILES
Blood glucose excursions were measured with the use of an isCGM: FreeStyle Libre®, Abbott. This glucose oxidase-based sensor was inserted thought a specific applicator into the subcutaneous tissue of the posterior part of the participant's arms, according to the manufacturer instructions. This device is capable of measuring glucose levels in the interstitial fluid every minute. The measurements are automatically stored in the monitor.
All participants were trained regarding the use of the isCGM. They were asked to take at least four glucose readings (before breakfast, lunch, dinner and bedtime) needed for the isCGM to store the whole data. Participants were also asked to insert information regarding meals and sleeping time. Moreover, they were asked to write down the glucose registered in the sensor before, 1, 2 and 3 hours after breakfast.
The investigators downloaded the data from the isCGM in the visits after the interventions. They set up software analysis period for the last seven days before the visit; the glucose target range was set up between 70 and 180 mg/dL. Participants were instructed to manually insert in the monitor the time they started the breakfast period.
VITAL SIGN MEASUREMENTS
Blood pressure (BP), heart rate (HR) and weight were measured once in all visits. BP and HR were measured after 5 minutes of quiet sitting, with participants with the feet on the floor, back in the chair, empty bladder, without eating, smoking or drinking coffee for at least 30 minutes.
STATISTICAL ANALYSIS
Based on previous isCGM studies in T2D patients (11), sample size was calculated in order to detect a 20 % reduction in iAUC with a power of 80 % and a significance level of 0.05. Anti-diabetic medications that act on PPG decrease the iAUC approximately by 20 %, and therefore this magnitude was considered to be clinically significative (12).
All data were assessed for normality and analyzed through the use of histograms and Q-Q plots. The isCGM variables presented a normal distribution, thus a paired t test was used to compare the standardized breakfast to the GTSS; these data are given as means + standard deviation (SD). The Mann-Whitney test was used to compare non-normally distributed variables, which are expressed as medians and interquartile ranges (25th-75th percentiles). Regarding vital signs, paired ANOVA test, with the Bonferroni post-hoc analysis, were performed to check if there were differences between visits, treatment groups and basal values. If a statistical difference was indicated, then, a paired t or Mann-Whitney test was performed separately with each pair.
The primary outcome measure was the iAUC of glucose concentration, which was based on the 3-h period after breakfast, represents the glycemic excursions following breakfast, of the 7-day PPG. The iAUC were calculated using the trapezoid rule. Baseline was defined as the pre-meal glucose value.
PPGE was calculated as the peak value of glucose after meals minus the glucose level at the beginning of each meal.
The statistical analysis were performed using GraphPad Prisma version 5.00.
RESULTS
Of the 36 participants enrolled in the study, two were screen failurea due to A1C levels outside the inclusion criteria. Three participants did not return to the visits: one of them did not want to continue due to difficulty to attend the visit; and two of them had problems with the sensor insertion and decided to quit. Therefore, 31 participants were submitted to both interventions. However, 3 of these participants did not have valid values regarding the iAUC curve in at least one of the interventions, so iAUC and PPGE were evaluated in 28 participants (Fig. 2). All of the other endpoints were analyzed considering the 31 subjects. No multivariate models were used because the lowest sample size number to be able to detect difference between the interventions was achieved. The intention-to-treat analysis was performed.
All the 31 participants reported that the entire content of the interventions were consumed.
BASELINE CHARACTERISTICS
Among the 31 participants age ranged from 39 to 69 years; most of them were women (58 %) (Table IV). The systolic BP range was between 100 and 169 mmHg; the diastolic BP between 69 and 109 mmHg; HR between 46 and 99 bpm; weight between 60 and 105.2 kg, and BMI between 22.04 and 34.91 kg/m2.
Table IV. Baseline characteristics of the participants.

BMI: body mass index; BP: blood pressure; bpm: beats per minute; HR: heart rate.
All of them have T2D; 22 (71 %) of them also have systemic arterial hypertension, 16 (52 %) dyslipidemia; 11 (35 %) have obesity (BMI above 30 kg/m2); 4 (13 %) lumbar pain; 3 (10 %) hypothyroidism, 3 (10 %) were tobacco users.
All of them were metformin users with a median dose of 1500 mg (1000-2000 mg). Average A1C levels were 7.5 ± 0.57 %.
isCMG
The iAUC was lower during the 7-days GTSS period compared to standardized breakfast period (33.3 [15.0-54.0] vs 46.8 [27.3-75.1], p = 0.0376) (Table V and Fig. 3).
Table V. Comparison between the GTSS and standardized breakfast regarding isCMG values.

MBG: mean blood glucose; PPGE: post-prandial glucose excursion; TIR: time in range.
*p < 0.05;
†p < 0.0001.

Figure 3. Mean blood glucose above basal glucose for the 3-h period after breakfast. N = 28 in both groups. Nutren Control® had a flatter curve and significantly lower iAUC compared to standardized breakfast.
PPGE was also lower during the 7-days GTSS period compared to standardized breakfast period (26.4 ± 17.2 vs 44.8 ± 24.4, p < 0.0001).
All the participants after 7 days of standardized breakfast had their post-prandial glucose peak in the 1-h value, while 5 participants (18 %) in the Nutren Control® period had this peak later than 1 h.
There was no difference between the intervention periods regarding MBG, TIR and hypoglycemic events (Table V).
VITAL SIGNS
Systolic BP was significantly lower in the visit after the 7-days period using Nutren Control® (127.1 ± 14.3 mmHg) compared to baseline values (137.9 ± 15.6 mmHg, p < 0.001), and also compared to the 7-day period after using the standardized breakfast (132.4 + 11.5 mmHg, p < 0.05). There was no difference between the baseline and the interventions regarding diastolic BP, HR, weight and BMI.
ADVERSE EFFECTS
Four participants (13 %) had mild diarrhea and 3 participants (10 %) had mild nausea in the Nutren Control group. The investigators considered these symptoms to be related to the GTSS.
COVID-19, flu, lipothymia and hypoglycemia occurred in one participant (3 %) during the period of Nutren Control® use, but the investigators considered it was not related to Nutren Control.
No adverse events occurred during the period of the standard breakfast.
DISCUSSION
This is the first clinical study to address the efficacy and safety of the recently designed diabetes specialized formula GTSS. In comparison to a standardized meal, the GTSS showed a 25 % reduction in the iAUC for the breakfast PPG. This positive glycemic impact showed statistical significance and was achieved following only one week of intervention, as seen in the isCGM downloads. Also, is of utmost importance to highlight that the standardized meal that served as our control intervention was in accordance with the American Diabetes Association nutrition guidelines (1) and with other important nutrition guidelines from societies in the field (2-4). The standardized meal, the first option to control the breakfast PPG, was isocaloric in relation to the GTSS group, which means that the main difference between the groups was the macronutrient distribution: the GTSS group had a higher protein percentage and consequently a lower carbohydrate content (Table III). In other words, a well-designed meal was compared to the supplement tested. These results were seen despite weight stability in both groups. Therefore, we could not attribute the glucose excursion impact to weight differences between the groups, thus presenting a “weight independent” benefit.
Other GTSS already in clinical use have shown similar results (12-23). In line with this work, Another GTSS was also tested as a meal replacement for the breakfast and produced an important AUC reduction in regards to glucose (12). When it comes to antidiabetic drug therapy, glipizide - a second-generation sulfonylurea - has produced a 34 % reduction in the glucose AUC (24). Other glucose lowering medications yielded impacts of same magnitude (25). Non-pharmacological strategies to treat DM are welcome in times of “patient-centered approach”. In general, people living with diabetes tend to adopt what they identify as less aggressive therapies.
In addition, an important point to consider is that the results observed were achieved on top of the use of metformin and in patients with a mean A1C of 7.5 %. In this common clinical scenario, the usual next step is to add another diabetes medication, an approach that could be easily substituted to a nutritional strategy ready to use - such as GTSS. As previously described (26) the lower the A1C, the higher the contribution of the PPG to the AIC level. GTSSs are intended to address eminently the PPG. As it is already known, the higher the A1C baseline, the better the results of the glucose lowering therapy. As such, the observed glycemic result in the iAUC was well above the expected.
There was also a small, but statistically significant, systolic BP reduction. This trend is also reported for other similar GTSS in the literature (4). This finding is coherent since improving nutrition, as a whole, impacts all cardiovascular risk factors such as BP and lipid profile (4). On the other hand, this was not the objective of the study, BP was measured only once in each visit and therefore this finding could be of random in nature.
The mechanism by which the GTSS impacts PPG is based on 4 major complementary factors: 1) its composition in terms of carbohydrates; 2) fiber content; 3) the presence of whey protein in considerable amounts of the protein blend; and to a lesser extent 4) the profile of fats in the formulation. The main carbohydrate component inGTSS formulation is isomaltulose, a complex carbohydrate derived from beetroot, which has a low glycemic index. A study by Maresch CC et al. (27) showed that in diabetic subjects, isomaltulose produces a 50 % lower AUC for blood glucose levels in comparison to sucrose. The impact of whey protein on glucose excursions, in regards of its insulinotropic effect, has also been a matter of scientific interest and it is nowadays well recognized. In the literature, some studies reported a A1c reduction of 0.9 % (28). Although commonly not properly addressed by health care teams, dietary fiber represents an important aspect in the diet of people with diabetes. In a recent meta-analysis by Reynolds AN et al. (29), incorporating adequate amounts of fiber in the diet of people with pre-diabetes, type 1 or T2D reduces 0,3 % of A1c. Ultimately, the lipid content in the supplement formulation it is expected to have some impact in terms of glycemic control. Ojo et al and Sanz-Paris et al performed meta-analysis that confirmed that diabetes specific formulas (with high mono-unsaturated fat content) produces a lower glycemic post-prandial response than non-specific formulas (with higher relative carbohydrate content) (30,31) The lipid content is expected to impact also the lipid profile as depicted by a meta-analysis by Garg A et al. (32). Garg A et al. pointed out a 4 mg/dL lowering effect on FG of high monounsaturated fat diets in people with T2D. At last, this type of nutritional intervention probably has its efficacy related not to one of its ingredients but to the whole composition.
Moreover, PPG in the GTSS group profile presented a smoother decay when compared to the standardized group. In other words, a flatter behavior. This effect is important for the proposed anti-hypoglycemia impact that GTSS is expected to produce. Although, this was not the scope of this trial, this distinction is probably due to the difference in terms of lipid and protein content from the two interventions.
In terms of safety, there were only minor gastrointestinal adverse events: 10 % of patients approximately had nausea and/or diarrhea, none of them leading to discontinuation of the tested supplement. It is important highlight that all patients had to be on metformin background therapy on study entry - a drug known to cause gastrointestinal side effects (26). The median metformin dose was 1,500 mg/day.
This trial had the objective of a first evaluation of this new GTSS and therefore has some limitations regarding the short and simple clinical trial that is worth mentioning: 1) the short period of follow up precludes us to extrapolate the findings to the long term; 2) the limited number of patients included in the trial; 3) the fact that it was conducted in a single center; 4) the results could be applied only in the selected population; 5) the absence of washout period. Besides the small number of participants and the subjects that were excluded due to invalid values regarding the iAUC curve sensor, it is important to note that the sample size calculations, done prior to de inclusion period, inform us that the size of our sample was sufficient to provide the statistical power needed to test the hypothesis. Moreover, even though the study was conducted in a single center, the population had a very mixed ethnic background with 35 % white, 23 % black and 42 % non-black/non-white. Nevertheless, the results obtained could not be extrapolated to other subpopulations of T2D, like those patients in use of more than one antidiabetic drug therapy, in therapy different from metformina, with different HbA1C levels and those with BMI > 35 kg/m2. The washout period has the objective to exclude the action of one therapy into the observation period of the next treatment. It is not expected that a food or the GTSS taken in the breakfast have some influence in the evaluated endpoints in the following day. For, example, there was no difference between the intervention periods regarding MBG and TIR, suggesting that the breakfast postprandial hyperglycemia does not affect the glycemia of the rest of the day. A more prolonged follow-up period could potentially clarify if these endpoints would be impacted by the intervention.
Other studies with this new GTSS should be performed in order to overcome those limitations: with longer period, with washout period and HbA1C as endpoint and with other T2D subpopulations.
CONCLUSION
The use of a diabetes specific liquid formula, as a meal replacement at breakfast, produced a 25 % reduction in the iAUC of the PPG as accessed by isCGM. This was achieved within a one-week clinical evaluation period and in comparison to an isocaloric standardized meal. This kind of nutritional supplement is a commonly neglected strategy in the treatment of diabetes and is the first study to point out that Nutren Control® can be used as a possible option for T2D patients not on glycemic target despite metformin use.

















