INTRODUCTION
Crohn's disease (CD) is a relapsing systemic inflammatory disease, mainly affecting the gastrointestinal tract with extraintestinal manifestations and associated immune dysfunction. In terms of etiology, CD is currently believed to be caused by both genetic and environmental factors. The environmental factors mainly include smoking and poor dietary habits and lifestyle (1). As economic and living standards have improved, the incidence and prevalence of CD have gradually increased. In Asia, studies have reported annual prevalence rates in Hong Kong and mainland China of 21.0/100,000 and 2.29/100,000, respectively (2,3). Inflammation of the digestive tract leads to pain, diarrhea, and nausea that, in turn, result in reduced food consumption together with erratic eating habits, eventually affecting the nutritional status of the patient; malnutrition is thus a common challenge in CD patients. The incidence of malnutrition varies with different nutritional screening tools and diagnostic criteria, with several studies suggesting that the incidence ranges from 20 % to 85 % in patients with CD (4-7). The European Society for Parenteral and Enteral Nutrition (ESPN) defines malnutrition as a condition in which a lack of or insufficient intake of nutrients leads to changes in body composition that result in physical and mental dysfunction and affect the clinical outcome of the disease (8).
Several studies have shown that dietary fiber, vitamin C, vitamin D, and vitamin E, amongst other factors, may have protective effects against the development of CD (9,10). However, there are few studies that have investigated the intake of different nutrients in patients with CD and there is also a lack of comprehensive analysis. Some foreign researchers have reported that, compared with the recommended dietary intake (RDI) of Canada, 18.7 % and 86.5 % of CD patients did not reach the daily recommended intake of protein and dietary fiber (9,11). In addition, over 40 % of the CD patients consumed less than optimal levels of vitamins A, E, C, folic acid, calcium, and zinc (11). Furthermore, other studies have reported that the adult average intake of calcium, magnesium, and zinc is lower than the RDI (12). In China, there are no reports on the comparative analysis of the diets and dietary components of CD patients compared with the recommended dietary intake for Chinese residents, and the relationship between the nutritional status of CD patients and the dietary components remained unknown. The assessment of nutrient intake may provide dietary guidance to CD patients to assess their nutritional status and lead to improvements in clinical outcomes. The purpose of this study was to assess the dietary nutrient intake of CD patients and explore the association between dietary nutrient intake and nutritional status, to provide evidence for proper nutritional support.
MATERIALS AND METHODS
STUDY DESIGN
A cross-sectional study was conducted at the Guangdong Provincial People's Hospital in Guangzhou, Guangdong Province, China. Sixty adult patients (ranging from 18 to 60 years) who had been diagnosed but had not yet begun treatment were selected between May 2018 and January 2021. The CD diagnosis was based on standard clinical, endoscopic, histological, and cross-sectional imaging criteria (13).
ETHICS AND PATIENT CONSENT
Permission from the Ethics Committee of the Guangdong Provincial People's Hospital was obtained before the study (No. GDREC2018091H). The clinical trial registration number was ChiCTR1800015174. The study was conducted according to the principles of the Declaration of Helsinki and the participants provided written informed consent.
MEASUREMENTS
Anthropometric assessments were performed in the morning after an 8-12 hour fast, and the same times of assessment were used for each participant. All assessments were conducted by the same investigator. Anthropometric measurements included height, weight, body mass index (BMI), mid-arm circumference (MAC), triceps skinfold thickness (TSF), mid-arm muscle circumference (MACM), handgrip strength of the non-dominant hand, and the left calf circumference (LCC) and right calf circumference (RCC). Body weights were taken to the nearest 100 g with an electronic scale; participants were barefoot and wore minimum clothing. A stadiometer was used to measure height to the closest 0.1 cm, and the BMI was determined as weight (kg)/height2 (m). BMI values less than 18.5 kg/m2 indicated malnutrition. The MAC and MACM were measured with a measuring tape at the mid-point between the olecranon process and acromion on the right side. TSF measurements were taken at the same position using Holtain skinfold calipers. Leg measurements included the circumferences of the thickest and thinnest parts of the calf, and the calf average measurement was calculated from three measurements. Nutritional status was determined by Patient-Generated Subjective Global Assessment (PG-SGA) with scores ≥ 4 indicating malnutrition.
Dietary recall (three days of 24 hours) was performed to record the nutrient intake of each person via face-to-face interviews. The patients were asked to describe the time, type, and amounts of all foods and beverages consumed over the previous three days. The interviewer used the food models to help the interviewee estimate the amounts of each food and beverage. The dietary information was recorded using a 24-h dietary review questionnaire. The date and the time of consumption of each food item were entered into the Nccw2006 Nutrient Analysis Software program. Macro- and micronutrients were calculated using the software, and the result was compared with the Chinese dietary reference intakes (DRIs). For energy, protein, zinc, vitamin A, thiamine, riboflavin, vitamin C, and niacin, intake values < 80 % of the recommended nutrient intake (RNI) were considered as not meeting the requirement. For calcium, phosphorus, magnesium, iron, and vitamin E, intake values less than the adequate intake (AI) value were considered to not meet the requirement.
STATISTICAL ANALYSIS
SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. For quantitative data, the Shapiro-Wilk method (W test) was used to assess the normality of the data distribution. Normally distributed data were described as means with standard deviations (χ̅ ± s), while if the data did not follow the normal distribution, the interval between the median and quantile [M(P25, P75)] was used for description. The form of the qualitative data adoption rate is described. Correlation analysis was used to analyze the relationship between nutritional status and various nutrients in patients with CD. All dietary nutrients were categorized into quartiles based on the distribution of the dietary intake of patients. Logistic regression analysis was used to calculate the odds ratios for the association between various nutrients and malnutrition risk-adjusted for age and sex. For analysis, the lowest intake of dietary nutrients was considered as the reference and the odds ratios in other quartiles were computed. All test level α values were 0.05, and bilateral probability was taken.
RESULTS
The anthropometric parameters of the study subjects are listed in table I. Forty-three males, accounting for 71.67 % of the total, and 17 females, accounting for 28.33 % of the total, were included. Twenty-eight patients had BMIs in the normal and underweight range, accounting for 46.67 % of the total number of patients, while there were two overweight and two obese patients, accounting for 3.33 % of the total number of patients. Nutritional status assessed by MAC, MACM, TSF, LCC, and RCC were all below the reference range of China. The average total score of the PG-SGA was 9.30 ± 4.77. Twenty-four patients had normal nutrition, accounting for 40 %. The number of participants with moderate and severe malnutrition was six and 30, respectively, accounting for 10 % and 50 %, respectively, of the total. Thus, in this survey, most patients exhibited severe malnutrition.
Table I. Characteristics of participants (n = 60).

BMI: body mass index; MAC: mid-arm circumference; TSF: triceps skinfold thickness; MACM: mid-arm muscle circumference; LCC: left calf circumference; RCC: right calf circumference; PG-SGA: Patient-Generated Subjective Global Assessment.
The dietary nutrient intake of the CD patients is shown in table II. Overall, 85 % of CD patients did not meet their energy requirements, and 63.33 %, 13.33 %, 11.67 %, and 100 % of CD patients had intakes of protein, fat, carbohydrate, and dietary fiber, respectively, that were lower than the Chinese dietary reference standards. The insufficient intake prevalence was 88.33 % for vitamin A, 90.0 % for vitamin B1, 91.67 % for vitamin B2, 76.67 % for vitamins C and E, and 50 % for vitamin PP. Overall, 21-100 % of CD patients did not meet their mineral requirements and 100 % and 50 % of them did not meet their requirements for calcium and phosphorus, respectively. The sufficient intake prevalence was 95 % for potassium, 55 % for sodium, 96.67 % for magnesium, 36.67 % for iron, 53.33 % for zinc, 91.67 % for selenium, 21.67 % for copper, and 65.00 % for manganese.
Table II. Dietary intake of patients with CD (n = 60).

CD: Crohn's disease.
*n(%) denotes numbers and percentages lower than the standard of Chinese dietary reference intake (2013 edition).
The results in table III show the relationship between the total score of the patients' PG-SGA evaluation scale and the intake of various nutrients. The statistically significant nutritional factors were energy, protein, carbohydrate, vitamin A, vitamin B1, vitamin B2, vitamin E, vitamin PP, and all minerals. In addition, the correlation analysis indicated that the total score of the PG-SGA evaluation scale was negatively correlated with the intake of each nutrient, that is, the higher the intake of each nutrient, the lower the total score of the PG-SGA evaluation scale, which represented better nutritional status.
Table III. Relationship between dietary intake and the PG-SGA scores of patients (n = 60).
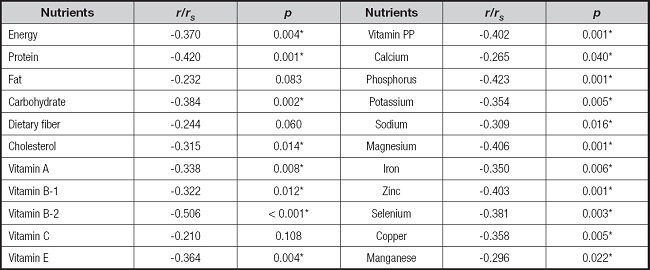
PG-SGA: Patient-Generated Subjective Global Assessment.
*p < 0.05.
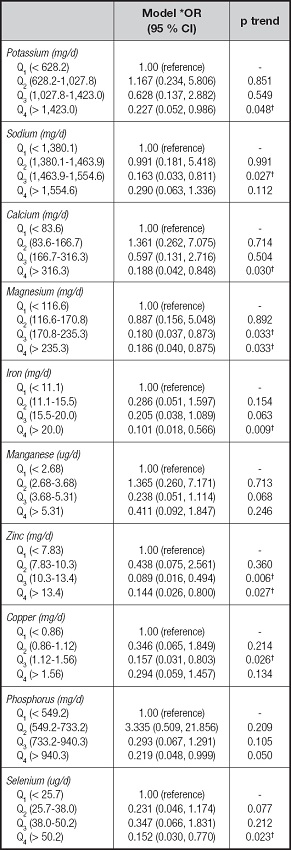
Table IV presents the odds ratios and 95 % CIs for the risk of malnutrition associated with energy, protein, fat, carbohydrate, and dietary fiber. For all patients combined, there was a significant decrease in the malnutrition risk among patients with CD in the third quartile (1,590.0-2,070.6 kcal/d for energy and 55.6-70.5 g/d for protein) intake of energy (OR = 0.050, 95 % CI: 0.009-0.279), and protein (OR = 0.150, 95 % CI: 0.029-0.773). We next examined the risk of malnutrition associated with the intake of individual vitamins, as shown in table V. In these patients with CD, inverse associations were observed for vitamin B-2, vitamin E, and vitamin PP, with evidence of significant dose response for vitamin B-2 (p trend = 0.002), vitamin E (p trend = 0.013), and vitamin PP (p trend = 0.002). For example, patients in the highest quartile of vitamin E intake had a significant 87 % reduction in risk when compared with those in the lowest quartile (OR = 0.125, 95 % CI: 0.024-0.640). Table VI presents the odds ratios and 95 % CIs for the risk of malnutrition associated with individual minerals. Comparing the highest to the lowest quartile of mineral intake, the odds ratios were lower for potassium (OR = 0.227, 95 % CI: 0.052-0.986; p trend = 0.048), calcium (OR = 0.188, 95 % CI: 0.042-0.848; p trend = 0.030), magnesium (OR = 0.186, 95 % CI: 0.040-0.875; p trend = 0.033), iron (OR = 0.101, 95 % CI: 0.018-0.566; p trend = 0.009), zinc (OR = 0.144, 95 % CI: 0.026-0.800; p trend = 0.027), and selenium (OR = 0.152, 95 % CI = 0.030-0.770; p trend = 0.023), whereas manganese was unrelated to the risk of malnutrition even at extreme levels of intake (OR = 0.411, 95 % CI = 0.092-1.847; p trend = 0.246).
Table IV. Logistic regression analysis of energy, protein, fat, carbohydrate, dietary fiber, cholesterol, and nutritional status in patients with CD.
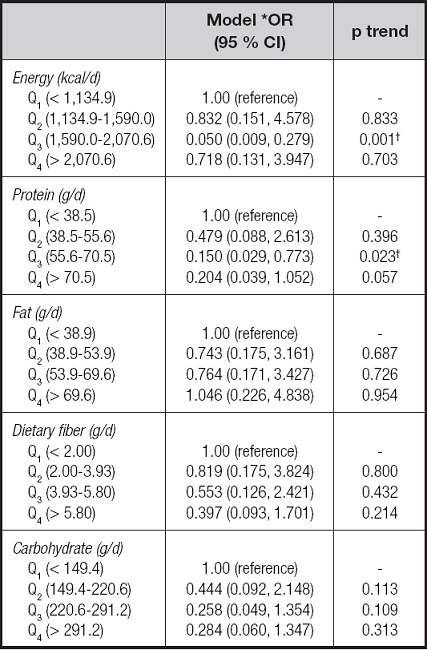
CD: Crohn's disease.
*Adjusted for age and sex;
†p < 0.05.
Table V. Logistic regression analysis of vitamins and nutritional status in patients with CD.
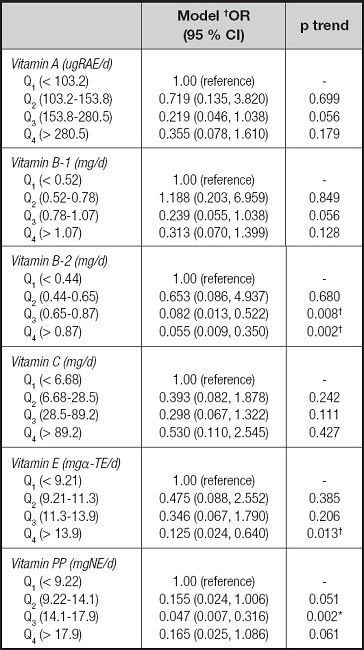
CD: Crohn's disease.
*Adjusted for age and sex.
†p < 0.05.
DISCUSSION
This study analyzed the nutritional status of patients with CD, finding an association between nutritional status and dietary intake. The PG-SGA evaluation scale was used to evaluate the nutritional status of CD patients. The results showed that the patients suffered from malnutrition to different degrees, with the incidence of moderate and severe malnutrition reaching 60.0 %, which is close to the results reported by Bian D (14). In addition, a systematic review concluded that close to one-third of CD patients have altered body compositions, seen as reductions in the BMI (15). The American Society of Parenteral and Enteral Nutrition (ASPEN) has proposed the use of specific parameters, including loss of weight, muscle mass, and subcutaneous fat, as well as handgrip strength, for diagnosing malnutrition (16). As shown by a recent systematic review, reduced muscle mass occurs in approximately 60 % of inflammatory bowel disease (IBD) patients (17). Body composition indicators such as reduced BMI and upper-arm circumference, amongst others, have been described in CD patients (18) who were also found to have reduced circumferences of the leg and mid-arm, as well as reduced mid-arm muscle (19). The results of our study showed that the mean BMI, MAC, MACM, TSF, LCC, and RCC values in CD patients were all lower than the corresponding reference values of normal Chinese adults. Altogether, malnutrition is a prevalent problem in CD patients and Coqueiro et al. concluded that deficiencies in nutritional factors in CD patients could lead to a risk of developing osteopenia and other complications in the long term (20).
In general, the incidence of malnutrition in patients with CD remains high, which may be caused by various factors (21,22). CD patients often experience abdominal pain, loss of appetite, nausea, vomiting, diarrhea, and other common gastrointestinal symptoms, which may lead to restricted diets and a reduction in food intake. In addition, reduced dietary intake can also lead to deficiencies in trace elements such as zinc and copper, which affect taste and will thus further affect their appetite. Furthermore, when the patient experiences active disease, although there may be increased nutrient consumption, the nutrient supplementation may be insufficient, further aggravating the occurrence of malnutrition. A study in Shanghai reported that people with CD did not receive sufficient energy, protein, calcium, and iron (21). Another study suggested that the intake of dietary fiber, as well as macro- and micronutrients, did not meet World Health Organization/Food and Agriculture Organization of the United Nations (WHO/FAO, 2003 edition) recommendations in most patients (23). However, there have been no reports of comparisons with the DRI standards for Chinese residents (24). The current study addressed the dietary intake of patients with CD. It was obvious that the dietary intake of most of these patients was far below the standard of the Chinese DRIs, and this, together with their low average food intake, may lead to further deteriorations in the nutritional status of patients with CD.
We found that the PG-SGA scale evaluations were negatively correlated with the intake of different nutrients. Similarly, a cross-sectional study (25) and an ambulatory study (14) also demonstrated a negative association between the PG-SGA and the dietary intake of energy and protein. These results indicated that CD patients having lower energy and protein intake may experience more severe malnutrition (reflected as higher PG-SGA scores). Thus, our research showed that an inverse association between dietary energy and protein intakes of 1,590.0- 2,070.6 kcal/d and 55.6-70.5 g/d, respectively, may reduce the risk of malnutrition in patients with CD. This, together with the findings that the energy and protein intakes in CD patients fell far below the DRIs, suggests that energy and protein should be increased appropriately to prevent malnutrition and to prevent disease progression.
In addition, vitamin deficiencies are often seen in patients with CD (26,27). Sufficient micronutrient intake is essential for improving the clinical outcome. Studies have shown that antioxidants such as vitamins A, C, and E can reduce oxidative stress, retard inflammation and delay the occurrence and development of diseases to a certain extent (10). B vitamins function as cofactors for essential metabolic enzymes and mediate fat, protein, and carbohydrate metabolism. B vitamins also play roles in both dietary and gut microbiome-mediated immune modulation. Moreover, B-vitamin supplementation in the diet in CD patients produces various anti-inflammatory effects and reductions in systemic oxidative stress and clinical symptoms, as shown by a clinical study (28). The results of this survey also showed that patients did not experience deficiencies in single vitamins but lacked multiple vitamins. The results of the current study suggested that higher intakes of vitamins B2, PP, and E from food were associated with a statistically significant reduction in the risk of malnutrition among patients. Therefore, attention should be paid to the supplementation of multiple vitamins in CD patients, such as at least 0.87 mg of vitamin B-2 and 13.9 mg-αTE of vitamin E daily, which may improve the body status of patients with malnutrition. At the same time, mineral deficiencies are also common in CD (27). Calcium and phosphorus play important roles in bone growth and development and CD patients have severe deficiencies in these minerals (100 % deficiency), which may increase the risk of osteoporosis in patients (29). Other minerals play important roles in tissue regeneration and protect cells from free radical damage, thus influencing the healing and prognosis of various diseases (30,31). In this study, we observed an association between the intake of individual minerals and the nutritional status of CD patients, with higher mineral intake reducing the incidence of malnutrition. Therefore, we speculate that appropriate supplementation of various minerals may be effective in improving patient nutritional status or delaying disease progression. Above all, we found that the average micronutrient intake was below the DRIs and, as dietary intake and nutritional status are correlated, clinicians should focus on micronutrient intake to ensure good nutritional status.
In this study, we analyzed the relationship between nutritional status and dietary intake in patients with CD, and found correlations between nutritional status and dietary intake, together with providing useful quantitative data that may reduce the risk of malnutrition among CD patients. Few studies have addressed this issue. Based on the results of this survey, we have several dietary recommendations for CD patients (32,33). It is recommended to take an appropriate vitamin and mineral supplement daily under the guidance of a doctor, including vitamins and minerals such as vitamins A, B-2, C, and E, and calcium, iron, and zinc, amongst others. In addition, if the patient is unable to improve their nutritional status from diet alone, enteral and parenteral nutrition could be used to provide supportive treatment.
The limitations to this study include the relatively small sample size, which precluded investigation of the relationships with sex and age using these as subgroups. Dietary recall, which was supposed to be three 24-h days, was not always accurate as, if a weekend intervened, some patients had difficulty in remembering food types due to the extended time. While we used PG-SGA for the assessment of nutritional status, we did not compare it with the ESPEN definition or the GLIM criteria. In addition, the lack of available equipment, such as dual-energy X-ray absorptiometry (DEXA) or bioelectrical impedance analysis (BIA), as well as nurses with professional training, was a non-negligible problem. Furthermore, the study was not able to assess causal relationships as it was not an intervention trial; cross-sectional surveys are unable to clarify cause and effect and can only suggest clinical significance. Future studies should use appropriately increased sample sizes for further analysis and provide detailed dietary guidelines for interventions.
CONCLUSION
In this study, the results showed incidence of moderate to severe malnutrition (reaching 60 %) in CD patients. The dietary survey found the intake of various nutrients by these patients fell far below the Chinese DRI standard, and there was a correlation between nutritional status and dietary intake. Appropriate intake of energy, protein, vitamin E, calcium, and other dietary nutrients may relate to malnutrition in CD patients. Therefore, early individualized nutritional guidance and suggestions may be beneficial for long-term improvements in nutritional status and may thus alleviate disease progression.