INTRODUCTION
Epidermolysis bullosa (EB) is a specific group of rare disorders presenting with fragility of the skin that are characterized by the formation of blisters with disruption of the dermoepidermal junction in response to minimal mechanical trauma (1). The disease affects individuals of both sexes and all racial groups, and an estimated 500,000 people are living with EB worldwide (2). In addition to the cutaneous manifestations, some EB subtypes present extracutaneous manifestations resulting in enhanced morbidity and negative effects on the patient's nutritional status. Recessive dystrophic EB — severe subtype (RDEB-S) is one of the types with the greatest nutritional impact (3), presenting with anemia, growth retardation, and impairment of the gastrointestinal tract, including constipation (4-6).
Patients with RDEB-S present hypercatabolism due the constant presence of skin lesions that increase energy, protein and micronutrient needs (6-8). Blood loss through wounds, chronic inflammation, and iron deficiency result in anaemia in EB (9). Low levels of zinc serum are present in the second year of life and this is associated with low weight in RDEB (9). Zinc levels can be falsely low in hypalbuminaemia, and assessment of zinc intake can be a factor contributing to its interpretation.
Some studies have evaluated the biochemical status of vitamins and minerals in EB with the intention of identifying deficiencies or excesses, both of which are important aspects of nutritional status. Giuseppe et al. evaluated, among other micronutrients, the levels of vitamins B6, B12, and serum folate in 20 children and adolescents with EB (10). Another study conducted exclusively in RDEB patients evaluated plasma levels of iron, calcium, folates, vitamins C, D, B12, A, E, B1, B6, and B2, zinc, selenium, and copper in 14 patients (11). Although biochemical status assessments are essential, monitoring biochemical markers in EB is difficult due to inflammation (9); monitoring nutrient intake becomes important and complementary to identify intake deficiencies and offers the advantage of being a non-invasive approach. In this regard, a few previous studies have evaluated the adequacy of nutrient intake in EB patients. Colomb et al. (12) evaluated energy intake in patients with RDEB, and observed that it was reduced to 56 % ± 18 % of the recommended daily allowance (RDA). Regarding the use of sip feed orally or via gastrostomy, the study by Yellet et al. (13) analyzed vitamin K intake and identified that patients who did not consume at least 200 ml of sip feed/day were at significant risk for deficiency. However, to our knowledge, no study has accurately assessed dietary intake in EB. Therefore, we aimed to investigate dietary intake including sip feed in a group of children and adolescents with RDEB-S to determine their intake adequacy for energy, protein, zinc, iron, and dietary fiber. Additionally, we sought to evaluate the clinical manifestations that may potentially affect food consumption.
MATERIALS AND METHODS
This cross-sectional analytical study evaluated children and adolescents aged up to 18 years with RDEB-S who attended an EB pediatric nutrition outpatient clinic. The individuals with RDEB-S participating in this study represent all patients in the age group followed up at our referral service for EB. Persons with EB are referred to our service by other hospitals or by the Society of EB Patients and Families. Signed informed consent and assent term were obtained from all parents and patients. This study was conducted in accordance with the Declaration of Helsinki (14), and was approved by the Ethics Committee of the Faculty of Health Sciences of the University of Brasília (registration number 1202012).
DEMOGRAPHIC PROFILE AND CLINICAL MANIFESTATIONS THAT AFFECT FOOD CONSUMPTION
The following information was collected from medical records and by using a structured questionnaire: demographic data, food consistency, feeding route, frequency of bowel movements, pain/difficulty in defecating, and presence of dysphagia, odynophagia, microstomy, difficulty in chewing, blisters in the oral cavity, ankyloglossia, gastroesophageal reflux, and pseudosyndactyly. EB type was based on previous clinical features and laboratory diagnoses.
RATIO OF SKIN LESIONS OF BODY SURFACE AREA
The ratio of skin lesions of body surface area (%BSA) was used in a formula to estimate energy requirements and was determined by filling a form (Fig. 1) containing a representative image of a person, back and front, that was subdivided into 100 small rectangles, each of which represented 1 % of body surface area. Form filling was performed at the time of dressing change. Parents and caregivers were instructed to color the areas with infected lesions and non-infected lesions in yellow and pink, respectively. To assess if the skin lesion was infected, the presence of some of the following signs was verified (15): 1) it becomes more painful, instead of gradually improving; 2) looks red around the skin edges, this red area may feel warm or hot; 3). looks swollen; 4) secretion of yellow material (pus), which may be smelly. The calculation of %BSA was carried out by the main researcher and was based on the sum of the areas shaded yellow or pink.

Figure 1. Guide to calculate the percentage of body surface area with skin lesions (adapted from: Haynes L, 2007 [7]).
NUTRITIONAL ASSESSMENT AND ESTIMATION OF ENERGY AND PROTEIN REQUIREMENTS
Assessment of nutritional status was performed using anthropometric data collected according the World Health Organization (WHO) criteria (16,17). The weight and height of the patients were measured by a trained professional. Length was measured with a horizontal anthropometer 110 cm long and with a 0.1 cm precision; In those patients aged two years or older a vertical anthropometer with an accuracy of 0.1 cm was used. A digital Filizola® scale was used for children younger than 2 years, with a maximum capacity of 15 kg and 10 g of variation. A platform-type Filizola® mechanical scale with a maximum capacity of 150 kg and a variation of 0.1 kg for children with 2 years or more. Due to the conditions of the patients, the dressings were not removed for measurements. In one of the patients, measuring height and weight using conventional methods was not possible due to contractures and dystrophy. Height was obtained with an inextensible millimeter tape, graduated every 0.5 cm, with the participant in the supine position; measures included the distance between the top of the head and hip, then from the hip to the knee, and from the knee to the base of the foot, and then these measurements were added. Weight was determined by measuring the weight of the child on the caregiver's lap, then measuring the caregiver's weight and subtracting the two weights.
For assessment of undernutrition in patients, the WHO indicators for underweight, stunting, and wasting were used (18).
Weight and height measurements were also used with a specific formula for children and adolescents with severe types of EB to determine their energy requirements (Fig. 2) (19). This formula uses current weight, energy for the corrected age, and additional factors such as %BSA, sepsis, and catch-up growth requirement. For %BSA one of the options of predetermined levels was chosen to be inserted in the equation — 0.19 for up to 20 % BSA; 0.5 for up to 40% BSA, and 0.950 for over 40 % BSA. Values 0.1 or 0.2 were used to determine catch-up growth requirements. To determine the degree of sepsis, the sum of the areas colored yellow was performed, and three levels of sepsis were considered with their respective values: mild = 0.2, moderate = 0.4, and severe = 0.8. The values corresponding to each of these factors are added into the formula according to the participant's condition.
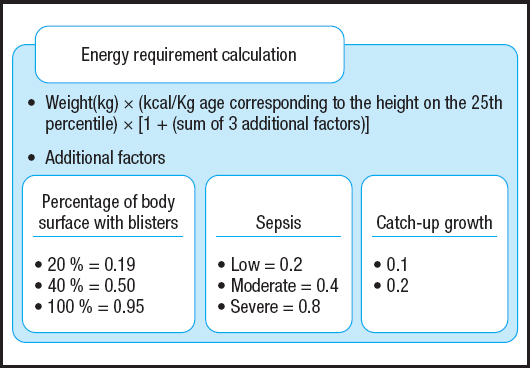
Figure 2. Formula to estimate the energy requirements of children and adolescents who had severe types of EB or were at nutritional risk (adapted from: Birge K, 1995 [19]).
To correct age, WHO growth standards (16) were used to identify the age corresponding to height in the 25th percentile for each participant. Subsequently, the amount of calories was determined from the tables of the Food and Agriculture Organization of the United Nations (FAO) according to sex and corrected age (20).
The protein requirement for patients was established within a percentage range of 115 %-200 % of the RDA according to sex and age (21). This percentage range is recommended for children and adolescents with EB (7).
INTAKE ASSESSMENT OF ENERGY, PROTEIN, ZINC, IRON, AND DIETARY FIBER
Seven consecutive daily food records (22) were used to determine the mean intake. The data were recorded by parents/caregivers. Comprehensive written instructions were given to parents/caregivers by trained professional, combined with face-to-face explanations to improve the quality of data for consumption of both solid, pasty, and liquid foods. For better control of effective food records, trained professional made telephone contacts on alternate days to clarify any doubts. Patients who used sip feeds were instructed to record them. And at the end of the registration period a trained professional reviewed the registration with parents/caregivers to clarify possible omissions and ambiguities, to probe for additional information, and to make any necessary changes. The intake of energy, protein, zinc, iron, and dietary fiber for each patient was calculated by the main researcher using the NutWin software (18). Food and sip feed that did not appear in the database were added by evaluating the food composition table (23) and food labels, respectively. Mean intake was determined by measuring the 7-day arithmetic mean.
We used the dietary reference intake (DRI) values (24) to evaluate the long-term mean nutrient intake described in the previous paragraph. For energy intake, we considered that children and adolescents with EB must consume an adequate amount of calories to facilitate healing and growth processes; thus, energy intake below the values calculated using the Birge formula was considered inadequate (19). For evaluation of protein intake, values within or above the reference range for EB patients (7) were interpreted as adequate intake, and values below the range were interpreted as deficient.
The estimated average requirement (EAR) was calculated for zinc and iron according to age and gender. For dietary fiber, we considered the adequate intake (AI) value since there is no EAR for dietary fiber. To accurately obtain the intake of iron, zinc, and dietary fiber without underestimation or overestimation, we considered a 10 % variability in the needs, and accounted for intrapersonal variability in nutrient intake based on the values provided in tables that estimate within-subject variations in intake. These values were obtained from a document issued by the Institute of Medicine, which provides guidance for application of DRIs in dietary assessment (24).
Using the variability values, the mean intake values, and the values for requirements (EAR/AI), as well as the adequacy of nutrient intake and the confidence of results, were determined. To calculate the adequacy of dietary fiber the AI was used and the 10 % variability in requirements was not considered, since AI represents the intake of a group and not a requirement. Confidence values equal to or above 0.85 were considered a low probability of inadequate intake. Notably, for dietary fiber intake values below the AI intake inadequacy could not be determined. We also determined the contribution of sip feed to the intake of energy, protein, zinc, and iron for each patient.
RESULTS
Seven patients with RDEB-S were evaluated in this study. All patients were diagnosed with undernutrition and five presented infected skin lesions. With respect to the clinical symptoms that affected food consumption, all participants presented at least three symptoms, namely, pseudosyndactyly, microstomy, and pain/difficulty in evacuation. Bowel movements were irregular in four patients, of which three patients reported defecation frequencies of 3 to 7 days whereas the fourth reported a defecation frequency over 7 days. All patients received only oral diets, and the consistency of their diets varied from liquid to normal (Table I). All patients completed the 7-day food record, and no meals were eaten away from home during the period. Due to their EB, the patients have an accompaniment at school, which could be their parents or a caregiver, which facilitated the registration. From our group of patients, the two newer ones, aged 1 year and 3 months and 3 years and 10 months, respectively, and the oldest participant were not attending school. Table II presents the individual values for energy and protein intake. To calculate energy requirement a value of 0.2 was considered for the catch-up growth item of all subjects, since all patients had low weight and/or short stature and needed a greater caloric intake to improve their nutritional status. While most patients had adequate protein intake, energy intake, in general, was inadequate. The use of sip feed, which accounted for 40 % to 50 % of their daily requirements, allowed only patients F01 and F04 to achieve adequacy of energy intake.
Table I. Food consistency, clinical manifestations that affected food consumption, stool frequency, and pain/difficulty in evacuation in children and adolescents with recessive dystrophic epidermolysis bullosa, severe subtype.
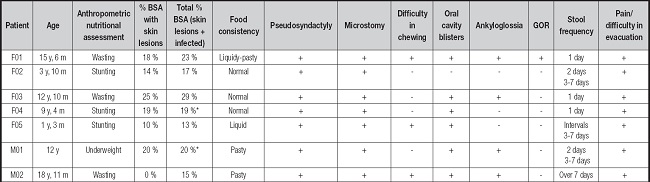
*All % BSA is non infected.
F: female; M: male; y: years; m: months; % BSA: the ratio of skin lesion of body surface area; +: present; -: absent; GOR: gastroesophageal reflux.
Table II. Adequacy of energy and protein intake, and the percentage provided by sip feed in children and adolescents with recessive dystrophic epidermolysis bullosa, severe subtype.
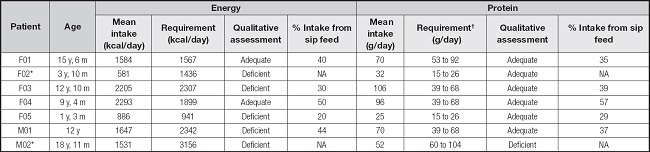
F: female; M: male; y: years; m: months; kcal: kilocalories; g: grams; NA: not applicable.
*Participant did not use sip feed.
†115 %-200 % of the recommended dietary allowance/DRI (EB requirement).
Most patients showed fiber intake below the AI (Table III), and only one participant showed low probability of inadequate intake (participant F03; 27.1 g). The intake of iron and zinc was adequate in most patients (confidence of adequacy: 0.98). The sip feed represented 20 % to 50 % of the patients' energy intake, 29 % to 57 % of their protein intake, 38 % to 75 % of their iron intake, and 42 % to 87 % of their zinc intake (Tables II and III). Notably, patient M02, who was the oldest participant, showed the maximal infection (%BSA, 15 %), wasting, deficient intake of energy, protein, and zinc, as well as no use of sip feed.
Table III. Adequacy of iron, zinc, and dietary fiber intake, and quantities provided by sip feed in children and adolescents with recessive dystrophic epidermolysis bullosa, severe subtype.
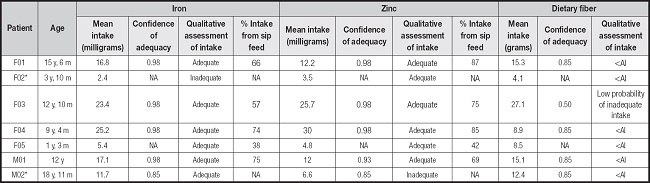
F: female; M: male; y: years; m: months; AI: adequate intake; <AI: less than the AI (adequacy of intake cannot be determined); NA: not applicable.
*Participant did not use sip feed. None of the patients used fiber supplements.
The adequacy of iron, zinc, and dietary fiber intake for two patients between 1 and 3 years of age was determined without considering intrapersonal variability in nutrient intake; as a result, the confidence values for this comparison could not be determined. This is because there is no variability in nutrient intake values available for this age group in DRIs.
DISCUSSION
Nutritional management is an essential component of long-term treatment for children and adolescents with RDEB-S, especially due the elevated risk of nutritional compromise. Adequate intake of energy, proteins, and trace elements is essential for growth, wound healing, and optimal immunity (11,25). Our results suggest that clinical manifestations that affect food consumption have a great impact on nutrient intake, and the use of sip feed can be a key management strategy to achieve nutrient intake adequacy and the primary nutritional approach to be employed in children with RDEB-S who are at nutritional risk.
To our knowledge, this is the first study to apply seven-day food records to establish the nutrient intakes in people with EB. This method is useful since the foods are noted at the time of consumption; records are not dependent on memory; and the types of food, preparations consumed and mealtimes are identified (26). The Institute of Medicine's documentation for the use of DRIs in dietary assessment of individuals highlights that data obtained over a greater number of days will yield more reliable results (24).
The factors that were shown to principally affect food consumption in the present study were similar to those reported in other studies. Food intake has been reported to be influenced by clinical manifestations such as oral and esophageal blisters, followed by scars that result in microstomy, ankyloglossia, and esophageal stenosis (4,6,25,27). In our study, the main factors that affected food intake were pseudosyndactyly, which impaired the ability to hold food and handle cutlery; microstomy, which makes it difficult to open the mouth; and blisters in the oral cavity, which caused discomfort, pain, and bleeding when chewing food. In the present study, all patients were fed orally since well-defined protocols for gastrostomy insertion in patients with EB have not been proposed in some countries. This factor may have played a decisive role in the patients' inability to meet their nutritional needs. Notably, the feeding route to be adopted in these patients should aim to mitigate clinical symptoms and ensure an adequate supply of energy, nutrients, and dietary fiber. Haynes (28,29) reported that nutritional sip feed with high energy and high protein content can be used in EB patients when natural food does not meet their nutritional needs; however, those studies also noted that the most compromised patients may be incapable of maintaining a satisfactory nutritional status despite efforts to maximize oral intake, needing enteral feeding. A study conducted at a referral center in Paris (12) found catch-up growth in children who received an average of 180 % of the RDA for protein intake through gastrostomy. A systematic review (30) evaluated the effectiveness of gastrostomy and concluded that it is a viable and safe alternative to improve the nutritional status and quality of life of patients with EB.
Energy intake is an important variable that interferes with protein balance, since insufficient energy intake can force the body to use the protein pool as an energy source (31,32). Patients with EB have greater protein requirements than their healthy peers due to the major loss of proteins through their skin lesions, the constant need for skin synthesis, and recurrent infections (33). Moreover, patients with RDEB-S are usually undernourished, as observed in the present study, and need more energy (7); in these patients, any deficit in energy intake in comparison with the protein-energy requirements may result in an increase in catabolism (8). The high values of %BSA with skin lesions indicate an increase in energy-protein requirements. We can raise some hypotheses with results of energy and protein intake: 1) considering that the currently protein recommendation for EB is reliable: diet (food and sip feed) ensured sufficient protein intake in these patients; however, did not provide enough energy, and protein intake is used to produce energy; 2) the protein recommendation that currently exists for children and adolescents with the most severe types of EB does not represent the real need, because these needs are difficult to establish due to variation in the extent and inflammation of lesions on a daily basis. Is a greater supply of protein necessary?
Difficulty was observed in the application of the specific formula to estimate the energy needs of patients since the interpretation of the items sepsis and catch-up growth is somewhat subjective. It is important to highlight that the term infection might be more appropriate to be used in the equation rather than the term sepsis; however, we decided to keep the name as in the original formula. Other more relevant situation is that establishing the energy and protein needs of children and adolescents with EB seems to be the biggest challenge, due to the interaction between the increase in nutritional needs, the emergence of clinical manifestations that affect food consumption, and the need for catch up growth. All these factors change constantly, and energy and protein requirements also change over a period depending on all these factors. A point that deserves further studies in EB is assessment of the individual's energy needs related to their mobility. It is well known that in some neurological diseases such as stroke, and musculoskeletal diseases such as arthritis changes in mobility occur (34). Efficient mobility is designed to minimize energy expenditure (35); however, in people with RDEB mobility difficulties are observed (36), which may be an indication of an increase in energy requirements. The formula for estimating the specific energy requirement for EB (19) does not consider mobility. Skin fragility, pain, joint contractures, fibrosis and fatigue are some of the many factors that contribute to changes in mobility in this population (37,38) and may impact energy requirements.
Iron and zinc are micronutrients that deserve special attention in EB, since their deficiency can increase susceptibility to infections and contribute to poor wound healing (39). In a study of seventy-three patients with several types of EB that included evaluations of the plasma levels of ten micronutrients, low levels of iron and zinc were found in those with junctional EB and RDEB. Ingen-Housz-Oro et al. (11) also reported iron and zinc deficiencies in a study of 14 RDEB patients. A retrospective study (9) that described the natural history of growth and anemia in children with EB identified low serum levels of iron and zinc between patients shows anemia in 91 % of RDEB patients and zinc deficiency in 55 % of RDEB patients. In this same study, significant positive correlations were found between weight in RDEB and levels of zinc and iron, which reinforces the need for monitoring nutrient intake and early intervention to minimize the impact of these deficiencies on growth.
It is known that the intestinal absorption of iron from the diet is related to the type of iron contained in the food, therefore the heme form of iron of foods such as meats is better absorbed than the inorganic form of iron found in vegetables and cereals. Children with RDEB in most cases have missing or malformed teeth, difficulty in chewing, ankyloglossia, esophageal stenosis, and fragility in the oral mucosa (40), and consequently meat is difficult or impossible to chew and swallow (28); therefore intake of heme iron from meats is scarce. In addition, malabsorption occurs as a result of severe recurrent denudation of the small intestine (4). In the present study, the intake of iron and zinc was adequate in comparison with the EAR for most patients, and this adequacy could be primarily attributed to sip feed. Thus, adequate assessment of the intake of these nutrients, as performed in the present study, is periodically recommended for show if the EAR is being reached and help establish the best supply of micronutrient to help minimize deficiencies. And must be associated with the periodic evaluation of biochemical tests.
Dietary fiber is also a prominent component for the management of RDEB-S patients (7,28,29). Constipation is a common clinical manifestation of EB and has been reported in all disease types, especially in RDEB (40 % to 75 % of cases) (6). Frequently, constipation is observed (4) in children/adolescents with different EB types that require laxatives and dietary fiber supplementation. In our study, most patients showed dietary fiber intake bellow AI, and none of them reported fiber supplement intake. In general, the consumption of fiber-rich food may be reduced because of the clinical manifestations that affect chewing and swallowing, as in the case of patients with EB. Thus, fiber supplements may be considered in these patients.
It can be difficult to obtain reliable anthropometric measurements from people with physical limitations and children with rare diseases who have inability to stand, contractures, scoliosis, lack of head and trunk control, among other reasons. An example is ankylosis and contractures of the lower limbs present in epidermolysis bullosa. We confirm in the present study that anthropometric measurements of weight and height in RDEB-S children represent an activity that requires a trained team, depending on the physical situation, and an alternative method of measurement may be necessary. In this study, to minimize potential errors in measurements, the same evaluator and the same equipment were used throughout.
At the time of data collection, our EB service was at the beginning of its structuring. Patients and family members contributed a lot to the team's learning. In this study, the form filling used to identify %BSA with skin lesions was performed by parents/caregivers. In EB and other rare diseases, many health professionals are not knowledgable of the disease and therefore, in many cases patients and their families have in-depth knowledge about their health care and treatment. According to the International Consensus on Skin and Wound Care in EB (37), people with EB and their caregivers are experts in the management of their condition and their involvement is paramount.
The present study had some limitations that require consideration. These include the small number of patients, a cross-sectional design that precluded cause-and-effect evaluations, and the non-inclusion of socioeconomic variables that may interfere with food consumption. Regarding the small number of subjects, it is important to note that EB is a rare disease and the real number of people with the disease in the world remains unknown. Only a few countries have adequate epidemiological data. In the United States, the incidence and prevalence of EB were estimated at 19.57 and 11.07 per million individuals, respectively. There are no epidemiological data about the incidence and prevalence of EB in Brazil; however, DEBRA-Brazil (Epidermolysis Bullosa Research Association of Brazil) has a record of more than 900 Brazilian patients. This number is likely to be significantly higher, considering the underdiagnosis of the disease and the lack of notification of new cases, especially in a country with continental dimensions like Brazil (41). Because our health EB service was still structuring at the time of study collection, some procedures that are currently routine for people with EB were not performed, such as wide and periodic biochemical evaluations and an esophagogram when stenosis is suspected. Regarding iron and zinc, we recognize that the absence of biochemical testing limits the assessment of micronutrient deficiencies. Therefore, we show the intake adequacy of these micronutrients by comparing to DRI. Although the method used in this study to assess nutrient intake is considered the gold standard to assess nutrient intake, some considerations must be made: the patient may change eating habits and may omit recording certain foods.
Despite the rarity of this genetic disease, and the small sample size and limitations of the services specialized in rare diseases in our country, it was possible to raise several points of discussion that suggest new studies in EB. In conclusion, the nutrient intake in RDEB-S patients in this study was characterized by adequate intake of proteins, iron, and zinc, of which a significant percentage is provided by sip feed. However, fiber and energy intake remained deficient despite the consumption of sip feeds, which could be primarily attributed to the clinical manifestations that affected food consumption. The findings of the present study underscore the importance of nutritional monitoring to assess intake and ensure sufficient nutritional support for patients with this rare disease, which is often interpreted as only a skin disease but has enormous clinical and nutritional repercussions.














