INTRODUCTION
The eating habits found in the majority of the population are mainly influenced by lifestyle in the contemporary world regarding the increasing intake of processed foods. This dietary pattern is composed mostly of saturated fatty acids and trans-fat, sodium, and simple carbohydrates, among which fructose stands out. The set of these components characterizes the westernized diet (1,2). Previous studies have shown that the westernized diet used in experimental trials with rodents during pregnancy and lactation favors the onset of chronic diseases in adulthood. These characteristics are related to increased body fat and weight, dyslipidemia, preference for palatable foods, insulin resistance, and/or glucose intolerance; besides, changes in the expression of neuropeptides responsible for appetite regulation have been documented (3).
More recently, not only the quality or quantity of what is ingested but at which times or intervals of the day it is ingested has also been shown to be of interest as a predictive factor of health or metabolic disorders (4). Homeostatic hunger has a daily cycle with peak times defined in humans (5) and animals (6). The establishment of this rhythm is associated with a central pacemaker at the suprachiasmatic nucleus (SCN) located in the hypothalamus. The SCN regulates physiological rhythms and peripheral structures. However, the physiological rhythms of peripheral structures are influenced not only by the central pacemaker but also by exogenous factors such as nutritional composition (7) and feeding time (8,7).
Feeding behavior is a complex process that involves endogenous interactions of each organism and species with its environment (9), going beyond the endogenous rhythm of feeding. In rats specifically, feeding behavior involves a sequence of procedures expressed by the animal that can be modified by factors such as perinatal malnutrition (9) or dietary composition, usually increased by greater lipid intake (10,11). The study of feeding behavior in rodents is carried out from the study of the Behavioral Satiety Sequence (BSS). This test allows to analyze the feeding behavior of the rat (12). Although it has been reported that malnutrition (10), early weaning (13), and acute consumption of the westernized diet (7) alters feeding behavior, it is still unknown whether this behavior is altered due to the association of a maternal westernized diet with time-restricted feeding in the adult life of rats.
Therefore, male rats were submitted to a feeding window of 16 hours (4 hours of the dark phase and 12 hours of the light phase) of the 24 hours cycle. This experimental model tries to mimic the lifestyle of the humans known as "night eaters". We hypothesize that dietary restriction by itself and/or the westernized diet ingested by the mother during pregnancy and lactation can cause excess weight and body fat, hyperglycemia, hyperlipidemia, and changes in the feeding behavior of the adult offspring. The exploratory aim of this study was to examine the repercussions of time-restricted feeding on feeding behavior and on some parameters of glycemic and lipemic metabolism of the offspring of adult rats whose mothers were fed a westernized diet during pregnancy and lactation.
MATERIALS AND METHODS
ANIMALS
The research was developed in the Department of Nutrition of the Center for Health Sciences of the Federal University of Pernambuco - UFPE, and in the Laboratory of Metabolic and Nutritional Diseases of the Department of Veterinary Medicine located in the Federal Rural University of Pernambuco - UFRPE. All experimental procedures were submitted to analysis and approval by the Ethics Committee on Animal Experimentation of the Federal University of Pernambuco (UFPE) under protocol number 0036/2016, and abide by the resolutions and guidelines determined by CONCEA (National Council for Animal Experimentation Control) (14).
Sixteen virgin females and eight adult males (Wistar rats, Rattus norvegicus) from the colony of the Department of Nutrition were mated in the proportion of 2:1 (female: male). After confirming pregnancy by vaginal smear and monitoring weight gain, pregnant rats were packed in individual cages and divided into 2 groups according to the diet received: control (standard chow diet) and westernized (experimental diet). The animals were maintained at room temperature (22 ± 1 °C) with controlled 12 hours dark/light cycle, and they had free access to a commercial diet and water ad libitum until the experiments began.
On the 21th day the offspring were weaned. Male pups were included in the experiment and started to receive a standard chow diet. On the 30th day of life, the animals were placed in individual cages for adaptation, and at 45 days of life the experiments were performed with the formation of the groups according to maternal diet and the dietary restriction (control = C, westernized = W, restricted control = RC, and restricted westernized = RW). From 60 days of life, the animals of the RC and RW groups were deprived of feed for the first 8 hours of the dark period (from 8 am to 4 pm of an inverted circadian cycle) and the groups C and W received a standard chow diet ad libitum throughout the experimental period. After 8 weeks of daily food restriction, a test of behavioral satiety sequence (BSS) was performed and the animals were euthanized by an overdose of anesthetics (ketamine, 80 mg/kg and xylazine, 10 mg/kg). Abdominal fat and the liver were removed and weighed. The experimental design is outlined in figure 1.
BODY WEIGHT AND FOOD INTAKE
Body weight evolution was recorded weekly for 10 weeks. The standard chow diet offered to the control group progenitors had 23 % proteins, 63 % carbohydrates, and 11 % lipids totaling 3.6 kcal/g throughout the experimental period. The westernized group received an experimental westernized diet formulated from an adaptation of the study by Ferro-Cavalcante et al. (2013) (15) until the end of the lactation period (21 days postpartum), composed of 21 % proteins, 45 % carbohydrates, and 34 % lipids, totaling 4.6 kcal/g. After weaning (postnatal day 22), all puppies were fed a commercial diet ad libitum. The amount of feed offered to the restricted groups was the same as in the control and westernized groups. Food intake (g) was obtained by the difference between the amount of food offered and the rejected food (4).
BEHAVIORAL SATIETY SEQUENCE
The analysis of the behavioral satiety sequence followed the method determined by Halford, Wanninayake, and Blundell (1998) (12). The animals were submitted to behavioral tests (BSS) after the feeding restriction period of 8 hours daily for 8 weeks when the animals presented an average of 120 days of life. The tests were conducted in animals submitted to 2 hours of fasting after turning off the lights of the experimentation room. The rats were monitored under low-intensity red light (< 5 lux) in the dark phase of the 24-hour photoperiod. A test meal was offered and feeding, cleaning, and other activities were observed over 60 minutes by a trained individual and recording the time in which each behavior predominated. All 60 minutes were recorded on a video camera to be subsequently examined by a second observer.
Behaviors were categorized as feeding (ingesting food, gnawing, chewing, or holding food in paws), drinking, active (exploring movements around the cage, rearing), grooming (body care movements with the mouth and paws), and resting (sitting or lying in a resting position or sleeping). Other measures scored from the behavioral observation of feeding were: meal duration (time in minutes over the entire monitoring period the animal was eating food), and feeding rate (amount of food or energy consumed/meal duration), that were relativized by kg/kcal of kg of body weight. To promote feeding, food was removed from the home cages at the beginning of the dark cycle (active cycle for rodents) and the presentation of the diet took place during 1 hour after this period registering all behaviors mentioned above. Food was weighed at the beginning and the end of each session.
BIOCHEMICAL AND BODY FAT ANALYSES
After BSS, between 130 and 140 days of life, the animals were euthanized. After fasted overnight, the animals were sacrificed by excess anesthetic to perform a median laparotomy. After reaching deep anesthesia, a vertical incision in the abdominal region was performed to collect blood samples (5 mL) through intracardiac puncture. Each blood sample was centrifuged at 2500 G/20 min to obtain the serum and then stored in a freezer at -20 °C for further analysis. Serum samples were obtained to determine biochemical variables (fasting glycemia, total cholesterol, low-density lipoprotein (LDL), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine). Biochemical analyses were performed at the Department of Metabolic and Nutritional Diseases of the Federal Rural University of Pernambuco through the use of an automatic Labmax 400® analyzer and Latest reagents®. The abdominal fat was removed and weighed on a mass scale® (model AL200 Marte; Rio de Janeiro, Brazil).
STATISTICAL ANALYSIS
For statistical analysis, the Shapiro-Wilk test was performed to evaluate data homogeneity; a one-way or two-way analysis of variance (ANOVA) test was performed followed by Holm Sidak´s or Tukey´s post-hoc test. The Graph Pad Prism 7 for Windows (GraphPad Software Inc., La Jolla, Calif., USA) was used for all groups and the values are presented as mean and standard deviation (SD); p-values < 0.05 were considered to be statistically significant.
RESULTS
BODY MASS AND FOOD INTAKE
At 60 days old the bodyweight of the animals before time-restricted feeding showed a difference between western groups (an increase of 13 %) and control groups (C = 246.8 ± 8.5; RC = 258.8 ± 32.2; W = 279.5 ± 24.6; RW = 278.6 ± 23.4 g, p = 0.0149) indicating the effect of maternal diet. At the end of the experimental period, body weight gain (g) was similar between control groups and westernized groups. However, we found differences between the RC (58.0 ± 23.5) and W groups (W = 94.5 ± 28.0; RW = 71.6 ± 29.7) (p = 0.047). Even so, the final body weight (g) of the W group was 17 % and 18 % greater than that of the C and RC groups, respectively (C = 319.4 ± 22.7; RC = 316.8 ± 28.8; W = 374.0 ± 25.4; RW = 350.2 ± 30.4 g, p = 0.0001). The RW group also showed greater body weight compared to C and RC in the 7th and 10th week, respectively (Fig. 2). The RM two-way ANOVA test showed interactions between diet and time (F(9, 99) = 2.4, p = 0.016), and isolated effects of diet (F(3, 33) = 9, p = 0.0002)) and time (F(3, 99) = 181, p < 0.0001). Therefore, regardless of the time elapsed, there was an effect of maternal diet from the first week of intervention until the end of the experimental period.

Figure 2. Weekly body weight of offspring according to maternal diet and time-restricted feeding in adult life (Groups [n = 10 per group]: C: control group; RC: restricted control; W: westernized group; RW: restricted westernized. RM two-way ANOVA followed by Holm Sidak´s post-hoc C test.*Versus C group, #Versus RC group [p < 0.05]).
Before the time restricted-feeding, the groups did not show any differences in total diet intake, neither in the light phase nor in the dark phase of the 24-hour cycle. However, during the restriction period, the restricted groups reduced the food intake (by 46 % for both restricted groups) during the 24-hour cycle (C = 26.9 ± 2.1 g; RC = 14.7 ± 2.5 g; W = 27.1 ± 2.4 g; RW = 14.7 ± 2.7 g, p < 0.001). This reduction was likely from the total amount ingested in the dark period (C = 6.1 ± 1.5 g; RC = 2.2 ± 3.8 g; W = 6.5 ± 0.7 g; RW = 2.4 ± 4.2 g, p < 0.001), since no changes were observed in the amount of food ingested in the light period (C = 2.9 ± 0.9 g; RC = 2.7 ± 0.8 g; W = 2.5 ± 1.42 g; RW = 2.5 ± 0.8 g, p > 0.05). However, it is highlighted that immediately after diet replacement, the restricted groups showed a "temporary hyperphagia", increasing by 29 % the intake of diet in the RC group compared to C (C = 5.1 g; RC = 6.6 g) and by 22 % in the RW group compared to W (W = 5.9 g; RW = 7.2 g), but this was insufficient to generate a total increase in the daily amount of energy.
BEHAVIORAL SATIETY SEQUENCE
The BSS was performed after 8 weeks of time-restricted feeding allowed to identify whether the nutritional manipulation at the beginning of life and/or the restriction of food in the dark phase altered the pattern of frequency, size, and duration of a meal of adult animals.
It was observed that the restriction of food and/or the maternal diet did not cause loss of the natural structure of the eating behavior characterized by an initial state of feeding followed by a transition between rest periods mediated by cleaning and exploration behaviors (Fig. 3). However, comparing RC with C, a phase advance was observed in the satiety point that occurred at 38 min in the RC group against 45 min in the C group (Fig. 3 C and D). However, this difference was not statistically significant when analyzed by sequence by time-bin (Fig. 3 B and D) — e.g., the comparison between RC and C did not show any significance (F(1, 16) = 3.3, p = 0.0891). However, significant mother western diet x time bin interactions were evident for the duration of eating in W group versus the C group during the fourth, sixth, seventh, eighth time-bins (F (1, 16) = 61, p < 0.0001) causing an advance in the transition from eating to resting (Fig. 3 E and F). Thus, the time spent in rest was increased in the W group compared to the C group during the seventh and eighth time-bins ((F (14, 154) = 3.3; p = 0.0002) (Fig. 3E). The comparison with other groups (RC, RW, W) did not show any differences between RC and RW, but a phase delay was observed in RW compared to C in the sixth time-bin (F(1, 12) = 46, p < 0.0001) (Fig. 3 G and A). The set of changes related was accompanied by an increase in feed rate and a decrease in meal duration relativized by body weight in all groups compared to control, and between the RW x W and between RW x RC groups (Table I).

Figure 3. Behavioral sequence of satiety of adult offspring from dams exposed to westernized diet or control in pregnancy and lactation, and with temporal restriction of food. Each period (12 times) of 5 min quantifies the duration of the behavior during total test time intervals (60 min). Columns represent the proportion of the total number of behavior observations per time interval (C [AB]: control group; RC [CD]: control group with temporal restriction of food; W [EF]: westernized diet group in pregnancy and lactation; RW [GH]: westernized diet group in pregnancy and lactation with temporal restriction of food. The line of intersection between resting and eating behavior signifies the point of satiety; RM two-way ANOVA followed by Holm-Sidak´s post-hoc test; p < 0.05).
Table I. Meal microstructure parameters of adult rats from mothers exposed to a westernized diet or controls during pregnancy and lactation and time-restricted feeding.

C: control group (n = 9); RC: control group with temporal restriction of food (n = 10); W: westernized diet group during pregnancy and lactation (n = 11); RW: westernized diet group during pregnancy and lactation with temporal restriction of food (n = 7). One-way ANOVA followed by Tukey's post-test (p < 0.05).
*Versus C;
†Versus RC;
‡Versus W.
Together, it was evidenced that both the maternal diet and the restriction of food caused alterations in eating behavior when compared with the control groups (C and W, reference). Thus, it can be inferred that the maternal westernized diet itself modifies in the long term the feeding behavior of the offspring, and that the changes in eating behavior due to temporal restriction varied differently between groups and were influenced by the maternal diet.
BIOCHEMICAL PROFILE AND ABDOMINAL FAT
The statistical analysis revealed a difference in fasting glycemia as a function of the gestation/lactation diet, but without effect due to dietary temporal restriction or in association with gestation/lactation dietary intervention (Table II). For triglyceride values, both groups with westernized diets (W and RW) showed high rates. Regarding the other biochemical parameters evaluated, there was no alteration either due to the perinatal diet or the temporal restriction of food. Regarding enzymes associated with liver damage, only ALT, more specific to liver tissue, was altered due to temporal restriction associated with perinatal diet (Table II).
Table II. Serum biochemical profile and abdominal fat pad of adult rats from mothers fed a westernized diet during pregnancy and lactation, submitted or not to time-restricted feeding.
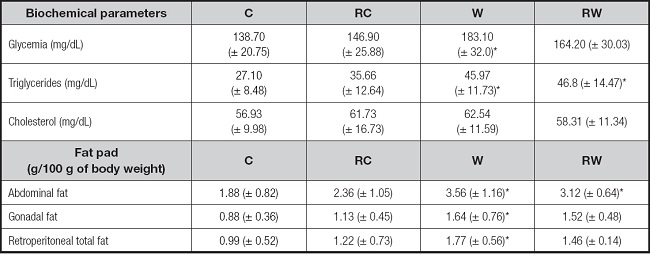
C: control group; RC: control group with temporal food restriction; W: westernized diet group during pregnancy and lactation; RW: westernized diet group during pregnancy and lactation with temporal restriction of food. One-way ANOVA followed by Tukey´s post-hoc test.
*Versus C versus RC (p < 0.05). Values expressed as mean ± DPM.
Table II also shows that the relative gonadal, retroperitoneal and total abdominal fat were higher in the westernized group compared to the control group, and the RW group exhibits a higher total amount of abdominal fat, but not compartmentalized between retroperitoneal and gonadal fat. No difference was observed in relative liver weight.
DISCUSSION
Eating behavior is a fundamental component in the control of the overweight/obesity processes because it can impact eating disorders (15) and physiometabolic disorders (16). The experimental model analyzed caused higher body weight and abdominal fat in the westernized groups. Interestingly, the time-restricted feeding did not promote weight or abdominal fat reduction between the restricted groups compared to their peer's ad libitum, even with restricted groups ingesting less food during the 24-h cycle. However, we highlighted the temporary hyperphagia found in both groups immediately after the end of the restriction period, and an alteration of the satiety point in animals from mother-fed westernized diet during pregnancy and lactation.
Similar responses to the weight increase of the adult offspring from mothers fed a westernized or high-fat diet were observed in previous studies (17,18). Possible changes in the energetic homeostasis of the offspring may be due to the phenomenon described as fetal programming, which shows that the conditions of the mother during the critical period of development will reverberate in the homeostasis of the offspring during adulthood (19). Disturbances in the energetic homeostasis of the offspring were later confirmed by other studies based on the use of a low-protein maternal diet (10), or high-fat diet (20) proving the consequences of the long-term maternal diet on the metabolic homeostasis of the adult offspring (21).
Food intake control involves a complex network of orexigenic and anorexigenic neuropeptides. TRF stimulates the secretion of orexigenic factors and causes animals to eat faster, suggesting that those factors act on mechanisms at the beginning and end of the meal (22). In rats, a high-fat maternal diet may result in changes in metabolic regulation and feeding behavior, resulting from a greater proliferation of orexigenic neurons (23) of the hypothalamus (24,25). These morphofunctional changes that occur in hypothalamic functions impact the body composition and glycemic control of the offspring (23).
The responses to the feeding behavior of the groups submitted to temporal food restriction varied according to the maternal diet. The group submitted to restriction from control mothers had an advanced point of satiety. On the other hand, the group of westernized mothers delayed the point of satiety when compared to their respective pairs of non-restricted controls. The behavioral analysis demonstrated that both the restriction of control and westernized diet affects the state of satiety observed by means of the feeding rate. This result can be explained by changes in the secretion of hormones related to hunger and satiety (25) with long-term effects on controlling food intake among other factors. In other fetal programming models such as low-protein maternal diet, the study of feeding behavior in the offspring demonstrated a delay in the firing of the satiety point in adulthood (10), similar to that found in young animals submitted to early weaning (13), whose models and results are opposed to those of the present study —i.e., the descending group of mothers fed a westernized diet (W or RW) demonstrated advancements in the firing of the satiety point, but both ate with more speed or a higher feeding rate. On the other hand, the RC group showed a slower feeding rate compared to the C group.
Altogether, we can infer that either due to lack or excess nutrients, the system of control of food intake in the adult offspring is strongly influenced by the maternal diet, and that deprivation time interferes differently according to composition diet. Moreover, we highlight that the results of our experimental design are pioneer in showing the influence of the westernized maternal diet on the feeding behavior of the offspring in the long term.
A cafeteria diet during lactation increases food intake in the adult offspring (26). The increase in food intake and consumption speed can be observed in our results. Westernized groups (W and RW) during BSS increased the speed of food intake by 100 % and 218 %, respectively compared to group C. The results of the maternal westernized diet also favor, in the adult offspring, an increase in ectopic fat deposits in the abdominal region, hyperglycemia, and hypertriglyceridemia, similar to those observed by Ferro-Cavalcante et al. (2013) (15). The increase in white adipose tissue deposits resulting from fetal programming has been translated by reduced fat oxidation in tissues such as skeletal muscle and brown adipose tissue (27,28). Therefore, there is a reduction in energy expenditure due to lower lean mass and altered mitochondrial metabolism (33-35). In part, this change seems to be associated with a reduction in the expression of decoupling proteins (UCPs) in brown adipose tissue, which plays a fundamental role in the thermogenesis process (30,31).
The increase in fat in the W group can be explained by metabolic programming, but the small fat reduction in the RW group was not expected. However, evidence from the literature has also shown that an increase in fat in the diet attenuates the amplitude of the clock genes and that temporal restriction restores this amplitude. A study conducted in 2012 showed that fasting at the beginning of the dark phase in mice resulted in an increase in the amplitude of lipogenic genes, resulting in new lipid synthesis in the liver, which is consistent with little reduction in abdominal fat and the hypertriglyceridemia (36) observed in our results in the RW group. However, it was observed in the present study that TRF seems to have minimized some negative metabolic effects generated by the maternal diet in the offspring, such as the restoration of glycemia, and not an elevation of retroperitoneal and epididymal fat.
In our study, a westernized diet caused changes in glycemia and lipidemia in line with the results found by Ferro-Cavalcante et al. (2013) (15), which constitute metabolic risk factors for associated diseases. Therefore, the nutritional composition of the dietary model used, associated or not with time-restricted feeding, is favorable to the development of disorders observed in the feeding behavior and metabolism of adult offspring. The westernized diet is characterized among other factors by having a high amount of lipids and fructose. The increase in fructose in the diet of rats is associated with the presence of glucose intolerance and hepatic steatosis (7,34,35). When present early in life, it seems to modulate epigenetic changes that predispose the individual to long-term metabolic diseases (36).
We recognize as a limitation of the study the absence of more in-depth molecular analyses such as the peptides related to feeding behavior, or the expression of clock genes. However, we revealed that this is a pioneering study in demonstrating the influence of both factors, maternal westernized diet and time-restricted feeding, on the risk of feeding disorders. This contributes to the establishment of links between obesity, eating disorders, and comorbidities. We also suggest that the results obtained herein can serve as a basis for subsequent studies to investigate the associated molecular machinery.
The set of results of the present study demonstrates that maternal diet and time-restricted feeding independently alter glucose and lipid metabolism and aspects of feeding behavior. From the BSS findings, it can be inferred that maternal diet influences in the long term the feeding behavior of the adult offspring, and that the time-restricted feeding affects the parameters of feeding behavior independently of the diet.
The importance that maternal diet during pregnancy and lactation has in the long term on the health of the offspring is well documented. This study shows that the feeding schedules of the adult offspring whose mothers had an obesogenic diet during pregnancy/lactation can impact the metabolic outcome and feeding behavior of these offspring in the long term. It is necessary that human studies be carried out to analyze the intensity of these effects on human health and, in this way, manage the consequences of a westernized maternal diet.















