INTRODUCTION
Aluminum contamination in parenteral nutrition (PN) solutions has been documented for decades. This can result in elevated blood aluminum, neurotoxicity, reduced bone mass and liver toxicity (1-3). Efforts have been made to reduce aluminum content in PN (1,4-6) particularly in the United States, where series of sills (toxic limit, upper safe limit and unsafe limit) for aluminum intake for patients on long PN have been determined.
The typical additives used in PN compounding have some degree of aluminum contamination. Multivitamins, trace elements, calcium, and phosphate salts all contain appreciable amounts of aluminum (4-6). The degree of aluminum contamination in a parenteral additive can be influenced by several factors, such as source of raw material and container design. Glass containers have notably more aluminum contamination than plastic ones. Data available related to calcium solutions used for PN suggested that glass-packaged calcium gluconate should be avoided as a component of PN solutions, and plastic-packaged calcium gluconate should be used instead, since higher amounts of aluminum contamination have been found in glass containers of calcium (4,5). Therefore, lower amounts of aluminum could be expected to be received by patients with multichamber-bag (MCB) PN compared to those with compounded PN, since MCB PN is a plastic-packaged PN. A retrospective study was performed to assess aluminum blood concentrations of patients receiving MCB PN compared to those receiving compounded PN.
The main objective of this study was to compare aluminum blood concentration according to type of PN administered, MCB or compounded PN. The secondary objectives were to analyze whether aluminum blood concentration may affect other biological functions such as liver and renal parameters, surgery, or admission to the intensive care unit (ICU).
METHODS
All hospitalized patients with PN, during the study period between 2015 and 2020, were eligible for participation in this retrospective study. Inclusion criteria for patients with PN were as follows: age ≥ 18 years and a blood aluminum measured as part of routine clinical monitoring during the hospitalization period. Exclusion criteria were as follows: patients under home PN therapy, and patients who had never had blood aluminum concentration measured.
The patients were identified from the pharmacy records of all patients to whom PN was dispensed. Anthropometric and clinical parameters, as well as indication, duration, type and amounts of PN, were collected from the pharmacy, medical, and nursing records. In addition to demographic, clinical and nutritional factors, data were collected on blood aluminum; renal and liver parameters were analyzed on the same day blood aluminum concentration was measured, as well as at baseline (start of PN). Eligible aluminum blood concentrations were those obtained during the PN and hospitalization period.
The PN formulations were either prescribed individually or as MCBs based on each individual patient's clinical condition and nutrition needs, maintaining the current clinical practice within the hospital (10). Following usual hospital practice, PN was prepared as “all-in-one” admixtures containing amino acids, glucose, lipid emulsion, electrolytes, vitamins, and trace elements. PN bags were administered in a 24-hour perfusion through central catheters or peripheral lines. Blood samples for aluminum determination were taken according to the hospital protocol for routine blood biochemistry determinations in hospitalized patients (at 7 a.m. during PN administration). PN was not stopped because of a blood draw. Indication for aluminum determination was at the discretion of the clinician in charge. For aluminum determination, blood samples were diluted 1:40 in 1 % nitric acid, and aluminum was measured using inductively coupled plasma-mass spectrometry (Nexion 350X instrument; PerkinElmer, MA, USA) with scandium as internal standard. The method was validated according to the European Medicines Agency (EMA) guidelines. Aluminum control material was obtained from ClinCheck® Recipe (Chemicals & Instruments GmbH, Munich, Germany) in line with the German Medical Association on Quality Assurance. The accuracy and precision of the method were verified using the following certified reference material for calibration: Multi-element Calibration Standard 3 PerkinElmer® (certified by EURACHEM ISO 17025). The limit of qualification was 1.48 µg/L, determined according to the EURACHEM guidelines, whereas linearity was from 1.5 to 250 µg/L.
In order to control and analyze factors that might affect aluminum blood concentration the following parameters were also collected: type of PN administered during sample extraction, liver (alanine aminotransferase [ALT], aspartate aminotransferase [ASP], alkaline phosphatase [AP], gamma-glutamyltransferase [GGT]) and renal parameters, number and type of PN previously administered during the hospitalization, renal replacement therapy (RRT), presence of hyperbilirubinemia (defined as total bilirubin ≥ 1.2 mg/dL), patient admitted to ICU and prior surgery during the same hospitalization period. Since ICU and surgery patients would be more likely to receive intravenous solutions, such as albumin and heparin (intravenous drugs with a higher probability of aluminum contamination), we chose these variables in order to control possible bias (1-3). Total bilirubin, liver and renal impairment were chosen because of their known relationship with aluminum contaminated intravenous administration (7-9). Samples were classified depending on the type of PN that was being administered while a sample for blood aluminum determination was drawn. Oral or enteral intake was not taken into account since aluminum is not expected to be absorbed by digestive route (1).
A second analysis was performed for long-term PN, defined as ≥ 20 days of PN. Since the PN protocol of our hospital promotes the use of standard MCB (10), very few patients receive only compounded PN during hospitalization. Therefore, in order to compare the effect of long PN on blood aluminum concentration according to type of PN in hospitalized patients, the results of patients with at least 20 days of PN were compared as follows: I) compounded group (patients receiving > 10 days of compounded PN), versus II) MCB group (patients receiving only MCB). The research protocol was authorized by the Ethics Committee of the Hospital, without the need for informed consent from patients due to the retrospective nature of the study.
The MCBs used were as follows: Olimel® N9 formulations (Baxter Healthcare Corporation, Deerfield, IL, USA) with electrolytes; Smofkabiven® formulations (Fresenius-Kabi, Bad Homburg, Germany) with and without electrolytes; Nutriflex® Lipid Special (B. Braun Medical city, Melsungen, Germany) with and without electrolytes, and Omegaflex® (B. Braun) with electrolytes. The macronutrient and electrolyte products used to prepare COBs were as follows: amino acids (Aminoplasmal-PO® [B. Braun Medical]); lipids (Smoflipid®, [Fresenius-Kabi]; and glucose (glucose 70 % 500 mL [Baxter]); water 500, 100, and 10 mL (Grifols); polyelectrolyte solution (polyelectrolyte solution for PN [Braun] 50 mL); potassium chloride 2M (5 mL, B. Braun Medical; 50 mL, Farmacia Carreras, Barcelona, Spain); magnesium sulphate 15 % 10 mL (10 mL, Genfarma, Toledo, Spain; 50 mL, Farmacia Carreras); calcium gluconate 10 % (10 mL, Suplecal®, B. Braun Medical; 50 mL, Farmacia Carreras); sodium acetate 1M (10 mL, B. Braun Medical; 50, mL Farmacia Carreras); sodium chloride 20 % (10 mL, B. Braun Medical; 500 mL, Grifols); and potassium acetate 1M 10 mL (B. Braun Medical). The vitamin and trace element products used for all PN in the study were Cernevit® (Baxter) and Addamel® (Fresenius-Kabi), respectively.
Descriptive statistics were carried out using frequency tables for all the variables. For continuous variables, descriptive statistics (mean ± standard deviation [SD]). For categorical variables, grouped percentages were used. Baseline characteristics and aluminum concentration of patients who received MCB PN and those who received compounding PN were compared using Student's t-test and Mann Whitney test in the event of an abnormal distribution, and chi-square test or Fisher exact test for categorical variables. To determine the relationship between the factors that might affect the aluminum blood concentrations univariate linear regressions were performed, with the factors assessed as independent variables and the aluminum blood concentration as a dependent variable. The clinical factors studied as independent variables were included in multiple-step regression models when they reached a significant level of p ≤ 0.1. Multiple linear regression was applied to determine the factors studied which are associated with aluminum blood concentration. All statistical analyses were performed with Stata software, release 13.0 (Stata Corporation, Texas, USA); and statistical significance is set at p < 0.05.
RESULTS
A total of 160 blood aluminum concentrations were available from 110 patients with PN during the study period. There was no difference in baseline characteristics between patients receiving MCBs of compounded PN, except for baseline AST and ALT (Table I). A total of 110 samples (68.8 %) were obtained during MCB administration and 41 samples (25.6 %) during the compounded PN administration. In addition, 9 samples (5.6 %) were obtained after the end of PN (mean days after the end of PN, 1.5 ± 0.6). No differences were found in blood aluminum concentration according to the type of PN administered (3.11 ± 2.75 µg/L for MCB vs. 3.58 ± 2.08 µg/L for compounded PN). Characteristics of all samples' values are presented in table II. Patients with RRT, surgery, baseline total bilirubin and hyperbilirubinemia showed higher blood aluminum concentrations (Table III). Likewise, there was also a positive relationship between the number of compounded PN received and blood aluminum concentration (coefficient B, 0.05 [95 % CI: 0.001 to 0.100]). The result from the multiple linear regression analysis showed that initial total bilirubin, surgery and number of PN bags administered were associated with blood aluminum concentration (Table IV).
Table I. Patient and PN baseline characteristics.
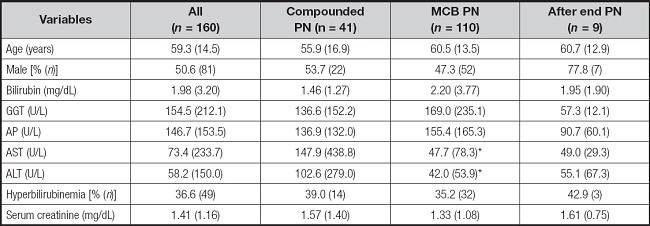
Data are presented as % (n) for categorical variables and as mean (SD) for continuous variables.
*p < 0.05 for significant differences between the compounded and MCB groups. GGT: gamma-glutamyltransferase; AP: alkaline phosphatase; ASP: aspartate aminotransferase; ALT: alanine aminotransferase.
Table II. Sample and clinical characteristics.
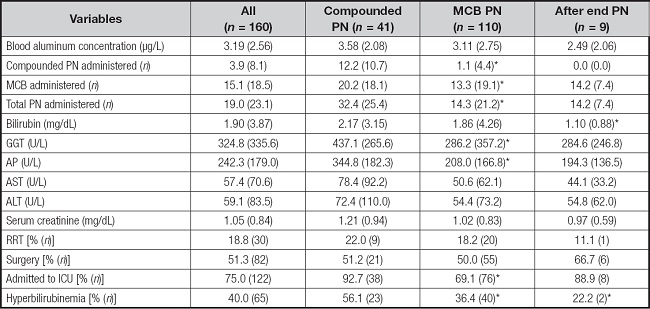
Data are presented as % (n) for categorical variables and as mean (SD) for continuous variables. RRT: renal replacement therapy.
Table III. Mean blood aluminum concentrations (µg/L) according to clinical conditions.
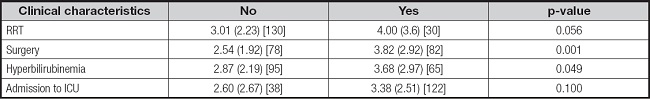
Data are presented as mean (SD) [n]. RRT: renal replacement therapy.
Table IV. Determinants of blood aluminum level by univariate and multivariate linear regression.
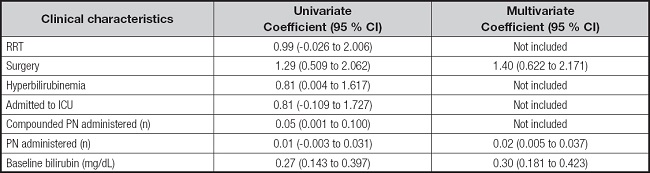
CI: confidence interval; RRT: renal replacement therapy.
A total of 38 samples were classified as long-term PN, defined as ≥ 20 days of PN: 21 samples after receiving only MCB and 17 samples were obtained after receiving at least > 10 com- pounded PN bags. Regarding blood aluminum concentrations in long-term PN, patients receiving only MCB showed lower blood concentrations compared to those receiving > 10 days of compounded PN (2.99 ± 1.55 versus 4.35 ± 2.17 µg/L, p < 0.05; Tables V and VI). The univariate analysis showed no relationship between any liver parameter and blood aluminum concentrations. Long-term PN therapy receiving > 10 days of compounded PN were related to higher blood aluminum concentrations (coefficient 1.36 [95 % CI, 0.09-2.63] for the univariate analysis, and coefficient 1.22 [95 % CI, -0.08 to 2.53] adjusted by days of PN).
Table V. Patients and PN characteristics for long-term aluminum determination and classified as compounded PN (at least > 10 compounded PN received).
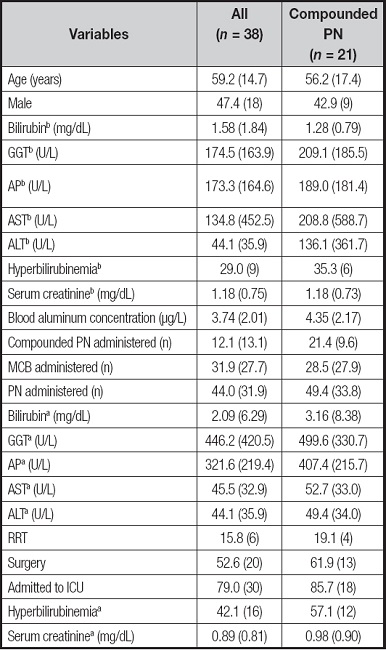
Data are presented as % (n) for categorical variables and as mean (SD) for continuous variables. bBaseline parameters. aCharacteristics when sample for aluminum determination was drawn. GGT: gamma-glutamyltransferase; AP: alkaline phosphatase; ASP: aspartate aminotransferase; ALT: alanine aminotransferase; RRT: renal replacement therapy.
Table VI. Patients and PN characteristics for long-term aluminum determination classified as MCB (all PN received was MCB).
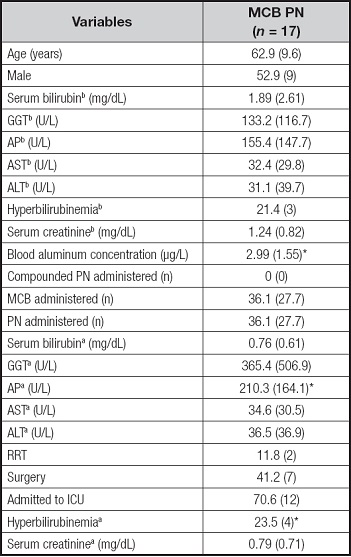
Data are presented as % (n) for categorical variables and as mean (SD) for continuous variables. bBaseline parameters. aCharacteristics when sample for aluminum determination was drawn. GGT: gamma-glutamyltransferase; AP: alkaline phosphatase. ASP: aspartate aminotransferase; ALT: alanine aminotransferase; RRT: renal replacement therapy.
*p < 0.05, significant differences between the MCB and compounded groups.
DISCUSSION
This study found no differences in blood aluminum concentration between patients receiving MCB PN and compounded PN. In addition, our study found that patients with longer PN therapy, hyperbilirubinemia and surgical patients showed a positive relationship with blood aluminum concentrations. However, hospitalized patients with long PN therapy receiving at least 10 days of compounded PN showed significantly higher blood aluminum concentrations compared to those with long PN therapy receiving only MCB PN regardless of surgery or bilirubin level, and keeping close to a significant difference when it was adjusted by total PN bags administered.
Recent reports have shown that long-term PN patients continue to be at risk of aluminum toxicity (3-5,11,12). There are several factors that might influence the degree of aluminum contamination in a PN additive. This work has studied the blood aluminum concentrations of patients receiving PN compounded from different sources: MCB PN and compounded PN. Apart from the package material of the MCB, it being plastic and not glass, fewer additions are needed in the compounding process with MCB PN. Hence, there is a lower probability of aluminum contamination. Long-term PN, renal impairment and RRT, as well as PN additives with glass containers have been related to a higher load of aluminum contamination. Similarly, this work found higher aluminum concentrations in patients with RRT, longer PN and PN composition with more PN solution additives as reported in previous studies (1-5,13). Since the establishment of the FDA aluminum mandate research has been conducted in order to determine whether PN compound with more recently manufactured products fits this mandate and whether they might affect aluminum levels in patients. This research found that the main sources of aluminum contamination were small-volume products used in the PN compounding process (such as multivitamins, trace elements, calcium and phosphate salts), albumin and heparin (1-5,12). Glass containers have more aluminum contamination than plastic. In addition, the type of fluid packaged in glass also influences the amount of aluminum contamination (1-3). Furthermore, other factors that impact the degree of aluminum contamination are storage conditions and long expiration dates. The longer a product stays in storage, the greater the degree of leaking aluminum from the glass vial into the solution. Accordingly, a reduction in aluminum contamination has been shown in calcium salt additives in plastic packaging compared to those in glass containers (4,5,12). Our results agree with the suggested idea that the MCB PN compounding process might lead to reduced aluminum contamination since lower blood aluminum concentration was found in long PN patients receiving only MCB compared to those receiving > 10 days of compounded PN. Several points support these findings, such as that fewer additions are needed with MCB, only multivitamins and trace elements if no electrolytes supplementation is needed and there is no glass contact during its storage (11).
Besides in the kidney, bones, and brain, aluminum is also accumulated in the liver. In fact, according to prior reports, accumulation in the liver happens before that in bones, kidneys, and the brain (12). In addition, it has been suggested that the contamination of aluminum in PN plays a role in PN-associated liver disease (PNALD) since parenterally infused aluminum might cause hepatic injury similar to that seen in PNALD (13-15). However, some works failed to show similar results, indicating no increase of liver parameters linked to parenteral aluminum administration (16,17). In this line, our results showed that blood concentrations of aluminum were not related to higher liver parameters. Nevertheless, initial total bilirubin and hyperbilirubinemia had a positive relationship with higher blood aluminum concentrations. Prior studies in piglet and rat models found a relationship between elevated serum bile and hepatic aluminum concentration in these animal models. Furthermore, piglet model studies also found a relationship between intravenous aluminum administration and decreased microvilli in bile canaliculi (7,17). Regarding our results related to initial total bilirubin and hyperbilirubinemia, since aluminum can be excreted by bile, we hypothesized that higher levels of total bilirubin may lead to impaired aluminum elimination by bile, and therefore, to higher aluminum blood concentrations (1,2). However, renal excretion should be taken into account, especially, when renal impairment is not present.
Despite this, the blood aluminum levels found might be considered to be low and within normal values. That might lead to think that no changes in PN were made because of the aluminum levels found. However, it should be remembered that aluminum has no known physiological role and therefore, no human being would be expected to have any amount of aluminum within them. In addition, similar blood aluminum levels have been observed in adult patients with RRT, reporting higher mortality in patients with blood aluminum concentrations ≥ 6 µg/L (13). The amount of aluminum that can be deposited varies with age. In neonates, close to 70 % of parenterally infused aluminum is retained, in comparison to 40 % in adults (2). Furthermore, blood concentrations have been shown to decrease over a period of PN administration where preterm neonates retained more than 50 % of the infused aluminum (18). However, animal model studies showed that despite adult rats not accumulating as much aluminum as newborn rats, aluminum levels in all tissues of newborn rats decreased after the end of exposure, while it continued to increase in adult subjects (19). The greater concern regarding aluminum toxicity is related to intake over longer periods of time, as well as vulnerable populations such as renal impairment patients and pediatrics, especially neonates. In light of our results, strategies to reduce aluminum load infused in patients with PN would include the use of MCB, especially in paediatric, but also in elder patients, renal impairment, hyperbilirubinemia and long-term PN. The use of MCB can also offer potential benefits such as lower costs, reduced time and labor, and fewer errors in PN preparation (14,20). Several studies have suggested that the needs of > 75 % of adult patients might be satisfied with standard MCB PN, including home PN (7,20,21). Moreover, currently pediatric guidelines suggested that standard PN should generally be used over individualized PN in the majority of pediatric and newborn patients (22).
Nevertheless, there are several limitations to this study. Eligible patients were only those with available blood aluminum data. Another limitation of this study is related to the variability in timing of measurement of blood aluminum concentrations as well as the fact that most of the patients received both types of PN, showing also variability in the number of compounding PN administered. This variability detracts from the ability to draw conclusions. It is also important to note the limitations inherent in the retrospective design of the study, like gathering data through a chart review process as well as the increased risk of selection bias. Moreover, since this is a single-center study, the external validity of our results would be limited. However, it is notable that the results presented here are consistent with data from previous studies. Moreover, to our knowledge this is the first study assessing the differences regarding aluminum contamination with the administration of MCB PN. This study may contribute to characterize the underlying mechanisms that might affect blood aluminum concentration and the subsequent induced toxicity, and provide a design for preventative strategies.
CONCLUSIONS
There were no differences in aluminum concentration according to the type of PN administered — compoundedg or MCB. Higher blood aluminum concentrations were related to PN therapy length, renal impairment, and hyperbilirubinemia. However, in long-term PN, the administration of MCB was associated with lower blood aluminum concentrations when compared to compounded PN.














