INTRODUCTION
The incidence rate of prostate cancer ranks second among all cancers worldwide. It is estimated that there will be over 1.4 million new cases and 375,000 deaths due to prostate cancer worldwide by 2020, making it the leading cause of death for men in some countries (1). At the same time, the incidence rate of prostate cancer varies greatly in different countries and regions. According to data from the International Agency for Research on Cancer from 2002, The United States has the highest prostate cancer incidence rate in the world [124.8 per 100,000 person-years]. China has the 170th highest incidence rate of prostate cancer in the world [1.6 per 100,000 men]. Other Asian nations with low incidence rates include Japan (12.6/105py, 114th), South Korea (7.6/105py, 134th), and Vietnam (2.8/105py, 161), among others. This tendency may be partially attributed to dietary changes. The typical foods that make up the Asian diet supply a wide range of antioxidants and phytochemicals, both of which have been shown to reduce the risk of prostate cancer. Several of these antioxidants have the potential to prevent the development of prostate cancer. This is due to the fact that oxidative stress brought on by reactive oxygen species and the loss of antioxidant enzymes may both contribute to genomic damage preceding prostate cancer (2-6).
Prevention could be an important means of limiting this burden, and dietary antioxidants could be a viable component of this effort. Antioxidants included in food, such as vitamins, folic acid, and selenium, have been demonstrated in a substantial number of clinical studies to successfully lower the chance of developing prostate cancer. Two significant randomized controlled studies, the Nutritional Prevention of Cancer (NPC) research, and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study, revealed that selenized yeast and -tocopherol both significantly reduced the incidence rate of prostate cancer by 63 % and 32 %, respectively (or vitamin E) (7-10). The inclusion of selenium, vitamin E, and beta carotene decreased overall cancer mortality, according to a large-scale randomized controlled study including several varied regimens. These clinical results, backed by preclinical and epidemiological data (11-18) demonstrate that using dietary antioxidants could effectively reduce the risk of prostate cancer. However, the results of three large-scale combined antioxidant intervention experiments show that antioxidant supplementation does not affect the risk of prostate cancer. Meanwhile, other studies support the idea that various types of vitamin E appear to affect prostate cancer differently. Therefore, γ-tocopherol may lower the risk whereas α-tocopherol may increase the risk. There is no strong evidence that selenium, vitamin C, or β-carotene has any beneficial effects on prostate cancer. As a result, there is much debate on how dietary antioxidants affect the risk of prostate cancer.
The effects of several therapies on an illness are compared using direct or indirect comparisons using network meta-analysis, which also estimate the rank order of each therapy (19). To examine the effects of various dietary antioxidants on the risk of prostate cancer, we conducted a network meta-analysis in the current study. The objective is to assess the results of these dietary antioxidants and offer clinical practitioners and patients evidence-based recommendations.
MATERIALS AND METHODS
This systematic review was conducted in accordance with a protocol that had been established in advance (PROSPERO CRD42022350572), and it has been reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension statement for systematic reviews that add network meta-analyses for healthcare interventions (20). We complied with the excellent research procedures described in the International Society for Pharmacoeconomics and Outcomes Research report on the interpretation of indirect treatment comparisons and network meta-analysis for healthcare decision-making (21).
SEARCH STRATEGY
From its inception through April 2022, the researchers in this study examined four electronic databases (PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science). The PICOS tool served as the foundation for the search strategy: (P) Population: Males; (I) Intervention: Dietary Antioxidants; (C) Comparator: Control group with placebo only; (O) Outcomes: Incidence rate of prostate cancer. (S) Study type: RCTs. The detailed search strategy is shown on table I (PubMed is used as an example).
INCLUSION CRITERIA
Be a phase 2, 3, or 4 clinical controlled randomized trials.
Published in the English language.
Experimental group with different dietary antioxidants as an intervention.
A control group with placebo only.
Men with no prior history of prostate cancer.
Being the outcome indicator the incidence rate of prostate cancer.
EXCLUSION CRITERIA
Research studies that lacked thorough or accurate reporting of their findings.
Research studies that were not randomized controlled trials (including quasi-randomized controlled trials, animal studies, protocols, conference abstracts, case reports, or correspondence).
Secondary follow-up article (not primary RCT article.
Studies conducted on patients who have already diagnosed with prostate cancer.
Studies where patients were treated with other interventions.
STUDY SELECTION
Using the literature management tool Endnote, the material was vetted and eliminated. The titles of the literature were first checked by researcher S.L. for duplication, non-randomized controlled trial studies, review articles, conference papers, protocols, and correspondence. Two researchers, S.L. and J.C., evaluated the literature abstracts in order to decide which works should be included and which should be excluded. Both researchers examined the remaining material in its entirety before selecting further pieces for inclusion. Both researchers separately reviewed the literature throughout this procedure, and then they compared the remaining material to see if it was the same or different. If it was different, a third researcher, Y.W., discussed and resolved the issue.
RISK OF BIAS IN INDIVIDUAL STUDIES
S.L and J.C. assessed the risk of bias (ROB) independently using the Cochrane Handbook version 5.1.0 technique for assessing bias in RCTs. The following seven areas were evaluated: a) random sequence generation; b) concealment of treatment allocation; c) and d) participant and personnel blinding; e) inadequate outcome data; f) selective reporting; and g) additional sources of bias. Trials were divided into three degrees of ROB based on the number of components for which high ROB was possibly present: high-risk (five or more components), moderate-risk (three or four components), and low-risk (one or two components) (two or less) (22).
The ethical approval of this meta-analysis was not needed because all data were extracted from previously published data.
DATA ANALYSIS
In the studies with dietary antioxidants as an intervention, all variables were dichotomous variables expressed as ratio (OR) and its ninety-five percent confidence interval. Due to the likelihood of discrepancies between studies, we selected a random effects model over a fixed effects model for analysis.
According to the PRISMA network meta-analysis (NMA) instruction manual, we conducted NMA aggregation and analysis using Stata software (version 15.1) and Markov chain Monte Carlo simulation chains in a Bayesian framework (23,24). If the p value is > 0.05 we will utilize the nodal approach to quantify and show the agreement between indirect and direct comparisons, determined according to the Stata software's instructions. The consistency check succeeds (25).
Network diagrams of various movement interventions are presented and described using Stata software. A separate motor intervention and a different control condition are represented by each node in the resulting network diagrams, and direct head-to-head comparisons across interventions are shown by the lines linking the nodes. The number of studies is proportional to the size of each node and the breadth of the connecting lines (26).
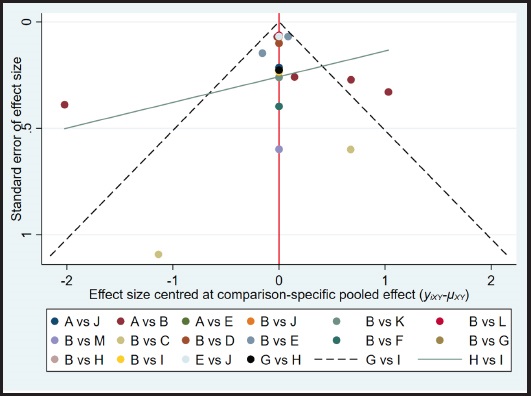
p score was used to summarize and describe the intervention hierarchy. The p score that assesses the degree of certainty that one therapy is superior to another treatment on average across all competing treatments is regarded as a frequentist equivalent to the surface under the cumulative ranking curve (SUCRA) values. The p score has a range of 0 to 1 with 0 being the worst treatment and 1 the best therapy with no ambiguity. Even though the p score or SUCRA might be helpfully re-expressed as the proportion of efficacy or acceptability of the exercise programs, such scores should be regarded cautiously unless there are genuine clinically significant differences across therapies (27). A network funnel plot was created and visually examined using the symmetry criteria to look for bias caused by small-scale research, which might result in publication bias in NMA (28).
RESULTS
STUDY AND IDENTIFICATION AND SELECTION
A total of 1783 documents were obtained from the electronic database. Duplicate papers were removed first, and the remaining 1063 documents were examined for titles and abstracts before 1023 documents were once again discarded. The remaining 43 documents were thoroughly studied, and 29 of them were once again removed (due to factors like inadequate data, conference papers, failure to meet the interventions contained in this review, and no meeting results included in this review), leaving only 14 documents for this study. The outcome is shown on figure 1.
QUALITY ASSESSMENT OF THE INCLUDED STUDIES
All studies were defined as low risk of bias. None of the experiments were described except one, which described a specific allocation concealment method, and all experiments included were randomized double-blind trials. The result shows on figure 2.

Figure 2. Quality assessment of included studies. Overall (left) and study-level risk of bias (below), using Cochrane's risk of bias assessment tool. Studies were deemed to be at high, low or unclear risk of bias based on adequacy of sequence generation, allocation concealment, blinding, method of addressing incomplete data, selective reporting, and other biases.
CHARACTERISTICS OF THE STUDIES INCLUDED
Overall, 14 RCTs were included in this study, with a total population of 73365 males. The intervention in the control group was mainly placebo, and the outcome indicator of the included clinical trials was the number of final prostate cancer cases. There were seven studies from the United States (5,29-34), two studies from Italy (35,36), one study from Norway (37), one study from Canada (38), and three studies are International Cooperative Research (39-41). Table II displays the characteristics of the studies included.Table II is not shown in the body of the manuscript as it is too large; it has been included in the supplementary material).
NETWORK META-ANALYSIS
Figure 3 will display the whole NMA figure.

Figure 3. Network of studies included with the direct comparisons available for primary efficacy outcome. The size of the nodes and the thickness of the edges are weighted based on the number of studies evaluating each treatment and direct comparison, respectively.
SUCRA efficacy charts
Figure 4 shows the efficacy curves of SUCRA for various interventions, with a larger area under the curve representing better efficacy. The results showed that GTCs ranked best in preventing prostate cancer incidence compared to placebo (SUCRA = 88.6) followed by vitamin D (SUCRA, 55.1), vitamin B6 (SUCRA, 54.1), and beta-carotene (SUCRA, 51.7). Selenium + vitamin E + isoflavones (SUCRA, 51.5). While other interventions were less effective than placebo, such as selenium + vitamin E (SUCRA, 50.8), selenium (SUCRA, 50.4), vitamin E (SUCRA, 50.0), folic acid + vitamin B12 (SUCRA, 46.8), folic acid + vitamin B12 + vitamin B6 (SUCRA, 46.5), selenium + vitamin E + GTCs ( SUCRA, 31.0), folic acid (SUCRA, 22.0). When we evaluated the comparative efficacy, GTCs were superior to all other drugs in preventing the development of prostate cancer.
The efficacy of dietary antioxidants in preventing the development of prostate cancer
Table III shows the efficacy of dietary antioxidants in preventing the development of prostate cancer. Comparisons should be read from left to right. The comparison's advantage ratios (95 % confidence intervals) are the same in the cells for the column- and row-limited treatments. For the prevention of prostate cancer development, a dominance ratio of < 1 favor row-restricted treatment. Regarding the risk of serious adverse events, a dominance ratio < 1 favors column-defining treatment.Table III is not shown in the body of the manuscript as it is too large; it has been added to supplementary material.
Table III. The efficacy of dietary antioxidants in preventing the development of prostate cancer.
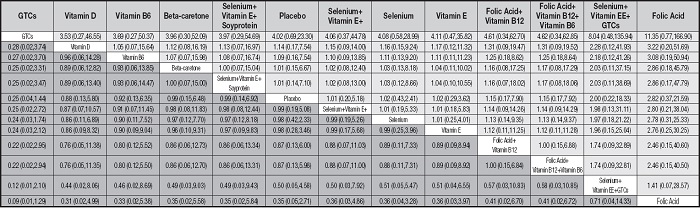
*A: selenium; B: placebo; C: green tea catechins (GTCs); D: vitamin D; E: vitamin E; F: folic-acid; G: folic-acid + vitamin B12; H: folic-acid + vitamin B12 + vitamin B6; I: vitamin B6; J: selenium + Vitamin E; K: selenium + vitamin E + isoflavones; L: beta-carotene; M: selenium + vitamin E + green tea catechins (GTCs).
DISCUSSION
In this research, we contrasted the effects of different dietary antioxidants on the risk of prostate cancer. A total of 14 studies were included in the article, at last, including 10 different intervention methods. The subjects included 73,365 men, which is a large sample size. The outcomes of our network meta-analysis indicate that green tea catechins (GTCs) could be the best dietary antioxidants to reduce the risk of prostate cancer.
Our results show that compared to other dietary antioxidants, green tea catechin has a statistically beneficial effect on the prevention of prostate cancer, and also a difference that is statistically significant compared to the control group. The main source of GTCS is green tea, which has obvious phenolic properties, and the (-)-epigallocatechin-3-gallate (EGCG) in green tea catechins is the main reason why green tea can prevent prostate cancer. Numerous fundamental studies have demonstrated that EGCG significantly slows PCa cell proliferation. Because EGCG may cause growth arrest in PCa cells as well as SV-40 immortalized prostate epithelial cells at dosages that have no harmful effects on normal human prostate epithelial cells. We should mention that the cytostatic activity of GTCs is cancer-specific (43-45). In a clinical randomized controlled experiment including volunteers with high-grade intraepithelial neoplasm of the prostate, green tea catechins were administered orally to inhibit the development of human prostate cancer. Sixty patients with high-grade prostatic intraepithelial neoplasm were randomly assigned to the intervention group, and the control group. The intervention group was given 600 mg GTCS daily, and the control group was given the same dose of placebo. The results of the study showed that among the 30 patients of the intervention group who received GTCs, 1 patient was diagnosed with prostate cancer, while 6 patients from the control group were diagnosed with prostate cancer. In another clinical trial on GTCS, the intervention group was given 400 mg of GTCS every day, and the control group was given the same dose of placebo. The results showed that there were fewer prostate cancer cases in the intervention group compared to the control group.
The results of our meta-analysis showed that compared to other dietary antioxidants, folic acid had no significant beneficial effect on the prevention of prostate cancer, and comparing the difference to the control group, it was not statistically significant. Whether folic acid prevents prostate cancer has been controversial, and the number of epidemiological studies investigating the effect of dietary folic acid or folate on the rate of prostate cancer is limited and the results vary. We found that four of them (32,46-48) showed a positive association, three (49-51) showed a negative association and seven (37,52-57) no association at all. In our network meta-analysis, prostate cancer risk was least affected by folic acid. In a randomized, double-blind clinical study, folic acid and aspirin were used to prevent colorectal adenoma. 32Jane C. Figueiredo conducted a secondary analysis of the results. After statistical analysis, the effect of aspirin on the rate of prostate cancer was excluded. Subsequently, 643 patients were divided into the intervention and control groups using randomization. The intervention group received folic acid supplement treatment while the control group received placebo treatment. However, the experimental results showed that the risk of subjects randomly assigned to the folic acid group increased significantly compared to the placebo group, and in the folic acid group, the estimated risk of receiving a prostate cancer diagnosis was 9.7 % (95 %CI, 6.5 % to 14.5 %) compared to 3.3 % in the placebo group within 10 years (95 %CI, 1.7 % to 6.4 %). The result of this study shows that folic acid not only did not reduce the risk of prostate cancer but increased its risk. significantly Another clinical randomized controlled experiment on folic acid showed that there was no significant difference in the prevalence of prostate cancer between the patients who received 0.8 mg of folic acid daily and those who received vitamin B6 and placebo.
In addition to GTCs and folic acid, our net meta-analysis also included clinical randomized controlled trials of other dietary antioxidants such as vitamin E, vitamin D, vitamin B6, selenium, and beta-carotene, five of which were associated with selenium. Selenium, an important trace element, is found in, at least, 25 different selenoproteins, such as glutathione peroxidas, an antioxidant enzyme that fights free radicals. Selenium has been studied as a potential anti-cancer agent since 1949, and estimates of dietary selenium intake have been connected to a number of malignancy death rates (58,59). Selenium supplementation has been found to lower the rate of some malignancies when combined with other antioxidant elements (60). The results of two of the five trials included in this paper showed a significant reduction in the incidence rate of prostate cancer in the intervention group relative to the control group; in contrast, two trials had a higher incidence rate of prostate cancer in the intervention group compared to the control group; and one trial had no significant difference in the rate between the intervention and the control groups was seen. The evidence provided by these studies on selenium and prostate cancer is limited and contradictory, and the possible reasons for this are somewhat related to ethnic and geographical differences, so we cannot be certain that there is a preventive effect of selenium supplementation on prostate cancer incidence.
Our study's key result is that, despite a large number of high-quality research, we are still unable to offer conclusive recommendations about the use of dietary antioxidants for the prevention of prostate cancer. This could be the result of our failure to appropriately classify patients, taking into account both the therapeutic risks and the advantages of dietary antioxidants. It will be crucial to stratify patients using new technologies as customized medicine and disease preventive studies enter a new age. To find commonly consumed dietary antioxidants, molecular phenotyping will aid early intervention studies that have been scientifically validated.
STRENGTHS AND LIMITATIONS
Our analysis included 73,365 participants from 14 trials, which is a fairly high sample size. Secondly, all the studies we included were 2-arm, 3-arm, and 4-arm studies that followed the principles of randomized control, and all the interventions in the control group were placebo, with a high level of evidence.
Our study has certain limitations that call for additional debate. First of all, conceptual heterogeneity in research designs, participants, treatments, or outcome measures poses the biggest risk to the validity of a network meta-analysis. By using strict selection criteria throughout the research's design phase, standardizing data abstraction, and contacting the study authors for any missing data to gauge the validity of our findings, we made an effort to reduce this. Secondly, subjects' adherence to the experiments varied among the trials, and therefore it was not possible to accurately quantify their impact on the analysis of the results. There were differences in the timing of outcome assessments that our analysis was unable to account for, and there were not enough data to conduct time-to-event analyses or calculate hazard ratios. Third, due to their very short duration and low possibility that patients would get the illness, several of the studies could not be evaluated for their effectiveness in preventing prostate cancer.
The reader should exercise care when interpreting the findings of our investigation since several of the studies were underpowered and certain therapies had scant evidence from head-to-head direct comparison studies. This emphasizes the necessity of expanding pertinent research further.
CONCLUSIONS
Our results suggest that GTCs are associated in intervention studies with a reduction in the incidence of prostate cancer and that it could be used as a dietary antioxidant in the daily recommended intake. Furthermore, given the low confidence level of some of the experimental results, molecular phenotyping, and more precise chemoprevention trials are needed to enhance the confidence level of the results in the future.