INTRODUCTION
Obesity is a major public health issue around the world. The World Health Organization (WHO) reports that since 1975, obesity has nearly tripled (1). Overweight and obesity, linked primarily to overconsumption of dietary energy, are strongly associated with an increased prevalence of diabetes, hypertension, and cardiovascular disease, as well as increased mortality from related non-communicable diseases (NCDs) (2).
It is now recognized that low-grade, chronic systemic inflammation is associated with most NCDs, including diabetes, obesity, cardiovascular disease, etc. (3). For a long time people have believed that adipose tissue's main function is to store energy; however, it is now known that adipose tissue also functions as a major endocrine organ that secretes adipokines, cytokines, and chemokines (4). Adipocyte hypertrophy in obesity is accompanied by disturbances in lipid metabolism and alterations in adipokine secretion, with a shift towards a pro-inflammatory phenotype (5). Adipokines like leptin and adiponectin are an indispensable part of the cascade of various metabolic and physiological signals, such as in the process of insulin signaling, glucose absorption, fatty acid oxidation, and other energy metabolism (6). Leptin is one of the main adipokines, and it influences multiple endocrine functions; it binds to specific receptors in the hypothalamic arcuate nucleus and suppresses appetite, thus playing an important role in body weight regulation (7). However, excessively increased circulating leptin makes the brain less sensitive to leptin leading to failure to respond, thus reducing leptin's ability to suppress appetite or increase energy expenditure, increasing food intake, and ultimately leading to overweight and obesity (8). In obese individuals, the overproduction of circulating leptin also greatly promotes a low-grade inflammatory state (9). Adiponectin, as adipokines, binds to receptors to promote glucose intake and prevent gluconeogenesis and fatty acid accumulation by activating AMPK, PPARα and other signaling molecules, thereby preventing insulin resistance and excessive accumulation of liver fat, and exerting anti-inflammatory effects (10). The adiponectin/leptin ratio has been proposed as a biomarker of adipose tissue dysfunction, showing a negative correlation with body mass index (BMI) and systemic inflammation (11,12). Cytokines such as interleukins (IL), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) also have important physiological functions and are involved in the generation, coordination, and cessation of immune or inflammatory responses (13). Persistent inflammation, whether due to prolonged exposure to stimuli or an inappropriate response to self-molecules, can lead to a chronic phase (14) characterized by sustained elevation of inflammatory cytokines.
Diet plays a significant role in modulating chronic inflammation (15). Dietary patterns can affect systemic inflammation, either directly or indirectly. Western diet is rich in red meat, high-fat dairy products, and refined grains, and is associated with high levels of inflammatory markers CRP and IL-6 (16). Conversely, a Mediterranean diet low in red meat and butter, and high in whole grains, fruits and vegetables, is associated with inflammation reduction (16,17).
The Dietary Inflammatory Index (DII) was developed to provide a quantitative means for assessing the role of diet in relation to health outcomes ranging from blood concentrations of inflammatory cytokines to chronic diseases (18,19). A negative DII score means the diet is anti-inflammatory. On the contrary, a positive score indicates that the diet is pro-inflammatory. Since its development the DII has been widely studied in various disease contexts to test the hypothesis that dietary inflammation is a determinant of NCD risk and mortality (3). A proinflammatory diet was significantly associated with a higher annual weight gain and a higher risk of developing new-onset overweight or obesity (20), while an anti-inflammatory diet, a healthy diet, and the consumption of healthy plant-based foods were all associated with a lower risk of developing obesity (21). In the cross-sectional Spanish PREDIMED (Prevención con Dieta Mediterránea) study, Ruiz-Canela et al. reported that the DII score was associated with higher average BMI, waist circumference and waist:height ratio in Spanish people (22). An intervention with an anti-inflammatory diet resulted in a significant reduction in body weight and visceral adipose tissue, and caused improvements in the participants’ cardiometabolic and inflammatory status (23). Therefore, it is necessary to study diet inflammation level in different obese populations from the perspective of prevention. A cross-sectional study showed that the prevalence of obesity was higher in the Uygur and Kazakh populations (24). Our previous study revealed that adiponectin and adiponectin/leptin ratio were inversely associated with metabolic syndrome (25), and that waist-hip ratio and leptin were negatively correlated with obesity in Uygur residents (26). It is unclear the relationship between DII and intermediate biomarkers in obese Uygur residents. Therefore, the aim of this study was to explore the association between a DII reflecting a more pro-inflammatory diet and adipocytokines among overweight and obese Uygur adults using a linear regression model.
MATERIALS AND METHODS
The current study included 283 obese and overweight Uygur adults according to inclusion and exclusion criteria. The data from a previous survey on dietary intake and obesity of local people in Hotan County during August 2018, Xinjiang. The inclusion criteria: body mass index (BMI) ≥ 24 kg/m2; 25 to 65-year-old Uygur residents who had lived in this area for at least 5 years, and agreement to participate in this study. All participants signed an informed consent. The exclusion criteria: pregnancy and lactation; inability to give their informed consent. The study was approved by the Ethics Cometittee of First Affiliated Hospital of Xinjiang Medical University [20170214-150].
Overweight/Obesity can be known by body mass index (BMI), which is calculated by dividing weight by the square of height. Overweight: 24 kg/m2 ≤ BMI < 28 kg/m2; obesity: BMI ≥ 28 kg/m2 (according to The health industry standard of the people's Republic of China - Determination of adult weight [2013 Edition]).
The general information (including age, gender, education, marital status, smoking and alcohol consumption habits, monthly income and self-reported physical activity) was obtained through face-to-face interviews.
Anthropometric indices were measured according to standard protocols, comprising height (accurate to 0.1 cm) and weight (accurate to 0.1 kg) under fasting conditions with light clothing and without shoes. All anthropometric measurements were done by trained personnel using calibrated instruments. In order to avoid the error of the results as much as possible, the same machine and standard were adopted by trained personnel in the measurement.
Dietary intake was collected using a validated and reliable 93-item food frequency questionnaire (FFQ), which was designed according to the local dietary characteristics and related references (27). The dietary intake data were collected by trained investigators and participants in face-to-face interviews.
Dietary inflammatory index (DII) is a literature-based tool that assesses the inflammatory properties of diet by reviewing 1,943 peer-reviewed articles that analysed 45 food variables and measured their associations with inflammation (18). The calculation of the dietary inflammation index can be divided into four steps: 1. Dietary intake data of participants were obtained through the FFQ. According to the 2016 Edition of the Chinese Food Composition Table, the dietary intake data were converted into the food composition content contained in the DII scale. 2. Dietary intake data of participants were compared with the global standard dietary intake database, which provide a reliable estimation of the mean and standard deviation, and the Z-score of each nutrient or food was calculated, i.e.: Z = (actual dietary intake amount - standard global mean) / standard deviation. Subsequently, to minimize the effect of “right-skewing”, the Z-value was converted into percentiles, while each percentile was multiplied by 2 and finally subtracted by 1. 3. Then the resulting value was multiplied by the corresponding food parameter effect score to obtain the food parameter-specific DII score for an individual (18). 4. Finally, all dietary parameter-specific DII scores were added together to calculate the overall DII score. The higher the DII score, the stronger the proinflammatory effect.
In the present study, a total of 22 food parameters were obtained by the FFQ, and used to compute DII (namely:energy, protein, total fat, carbohydrate, cholesterol, dietary fiber, vitamin A, thiamine, riboflavin, niacin, vitamin C, vitamin D, vitamin E, folic acid, vitamin B6, magnesium, iron, zinc, selenium, garlic, onion and pepper). The DII was divided into three tertiles, with first (< 1.15), second (≥ 1.15 and < 1.95), and third (≥ 1.95) being the most anti-inflammatory, neutral, and pro-inflammatory tertile, respectively.
The biochemical analyses were conducted on venous blood samples that had been collected after a 12-hour fast. The serum levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), and fasting insulin (FIN) were detected using an BS-460 and BS-800M automatic biochemical analyzer (Shenzhen Mindray company); leptin (LEP), adiponectin (ADPN), tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP) were measured by the enzyme-linked immunosorbent assay (ELISA) method with an appropriate kit (Elabscience Biotechnology Co.,Ltd). The ADPN/LEP ratio was calculated as the ratio between serum concentrations of adiponectin and leptin. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the formula: [(fasting glucose (mmol/L)) × (fasting insulin(ml/U))] / 22.5).
All analyses were performed using the SPSS software (version 25). The normality of quantitative variables was assessed using Q-Q plots and the Kolmogorov-Smirnov test. Normal quantitative variables were presented as the mean ± standard deviation (SD), skewed and categorical variables as frequency and percentage, and continuous variables as median and P25, P75. The relation between the DII score as independent variable and adipocytokine concentration as dependent variable was tested by linear regression (Model 1, unadjusted). Model 2 represents a regression analysis with adjustment for age, gender, and BMI. p values of < 0.05 were considered to be statistically significant.
RESULTS
Among the 283 adults, 193 women and 80 men, 103 overweight and 180 obese, mean age was 46.40 years. The mean DII was 1.35 (SD = 1.08), ranged from -2.14 (most anti-inflammatory) to 3.11 (highly pro-inflammatory). The participants’ characteristics are summarized in table I. Overall, those participants with high pro-inflammatory diet (third tertile) were more likely to be female, married, less educated people.
Table I. Characteristics of participants according to the tertiles of DII [n (%)].
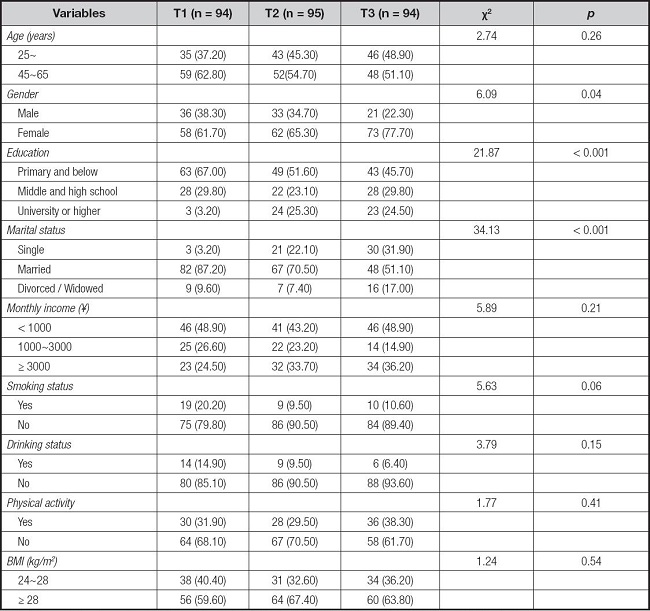
BMI: body mass index; Overweight: 24 kg/m2 = BMI < 28 kg/m2; Obesity: BMI = 28 kg/m2.
T1: < 1.15, 1.15 = T2 < 1.95, T3 = 1.95.
The dietary intakes are indicated in table II. Those participants with a lower DII score had higher intakes of anti-inflammatory diet components, such as dietary fiber, thiamine, riboflavin, vitamin A, B6, C, D, E, zinc, magnesium, selenium, niacin and folic acid.
Table II. Comparison of dietary intake of participants according to the tertiles of DII (M [P25, P75]).
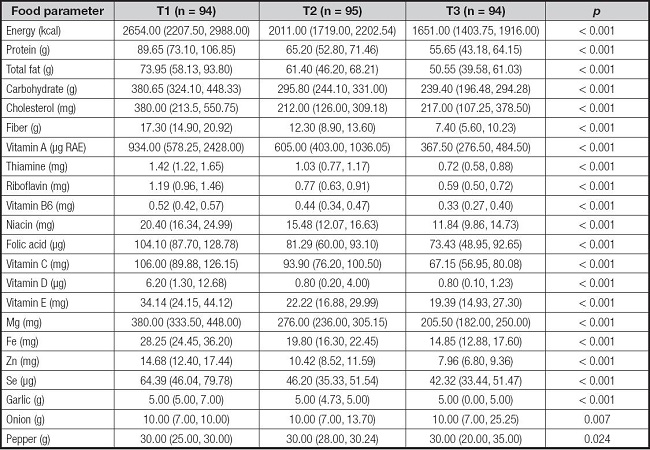
Data were reported as median (interquartile range) and analyzed using the Kruskal-Wallis H-test.
The β coefficient and standard error (SE) of correlation between DII and adipocytokines are shown in table III. The DII score is a significant reverse trend between HDL-C in the unadjusted model (β = -0.12, SE = 0.05, p = 0.02), and this remained after adjustment for age, gender, and BMI (β = -0.12, SE = 0.05, p = 0.02). Moreover, the DII score was positively associated with the LEP in the unadjusted model (β = 2.35, SE = 0.56, p < 0.001) and adjusted model (β = 1.64, SE = 0.53, p = 0.002). In addition, there was a negative relationship between ADPN and DII after controlling age, gender, BMI (β = -203.15, SE = 96.48, p = 0.04). The adiponectin/leptin ratio was negatively correlated with DII in the unadjusted model (β = -62.16, SE = 24.29, p = 0.01), but this relationship was not observed after controlling for age, gender, and BMI. And no statistical association was observed between DII and other biochemical indices in both the unadjusted and adjusted models.
Table III. Standardized regression coefficients (ß) and their standard error (SE), and p-value of the association of DII score with BMI and biochemical measurements.
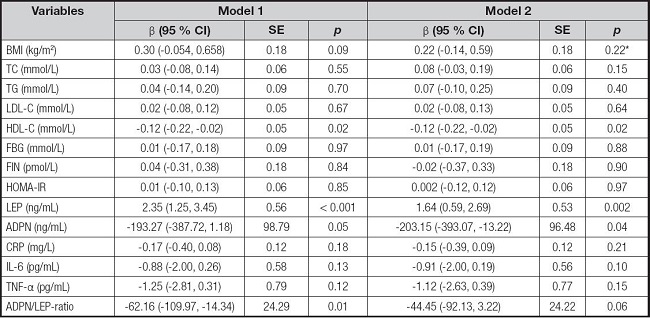
Model 1, linear regression analysis without adjustment; Model 2, linear regression analysis with adjustment for age, gender, and BMI or without BMI*.
DISCUSSION
Chronic inflammation can lead to various metabolic diseases including obesity through the influence of diet, and DII is a new method to evaluate the characteristics of dietary inflammation. Our findings showed that DII score was positively correlated with the concentration of leptin and negatively correlated with adiponectin in this studied obese Uygur adults. In addition, the DII score was inversely related to high-density lipoprotein. We did not find CRP, IL-6, and TNF-α to be correlated with DII score.
DII plays a very practical role in assessing the inflammation potential of diet. In our study, the positive association between DII score and leptin concentration suggests a role for the inflammatory properties of the diet in regulating adipose tissue inflammation. Considering nutrients, as with other studies (28,29), in this study DII was significantly inversely related to intake of dietary fiber and various specific nutrients (thiamine, riboflavin, vitamin A, B6, C, D, E, zinc, magnesium, selenium, niacin and folic acid). All these associations matched the DII's expected direction. However, it should be noted that T3 (with a highly pro-inflammatory diet) had relatively low intakes of energy, protein, total fat, carbohydrates, cholesterol and iron. Maryam et al. (30) reported that there were significant decreasing trends in the proportion of energy intake from carbohydrates, proteins and iron across categories of the DII score (from quartile 1 to quartile 4). Asadi et al. (31) also reported that participants in the third group of DII had a lower energy intake. These results are consistent with the results of this study. This may take into account the demographic characteristics of the participants as well as their dietary habits, such as according to the group of DII, it can be seen that there are more obese people in the T3 group. Obese people may have the intention to control their dietary intake to achieve weight loss.
Obesity and obesity-related diseases are closely connected to the serum levels of leptin and adiponectin (32). The majority of obese individuals show leptin resistance, characterized by abnormally increasing serum leptin but diminished effects of leptin on inhibiting appetite and enhancing energy expenditure, which causes an increased food intake (8,33). Leptin also stimulates the production of proinflammatory cytokines IL-6 and TNF-α, and promotes inflammation (9). In this study, the DII score was shown to be positively associated with leptin concentration after adjusting for age, gender, and BMI. The same was observed in other studies in which there was a significant correlation between plasma leptin concentration and DII score (β = 0.096, p = 0.020) (34).
Adiponectin has insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects, and, in certain settings also decreases body weight (9,35). In our study there was a negative correlation between DII score and adiponectin concentration in the adjusted model. These results indicated that an anti-inflammatory diet is associated with a lower risk of developing obesity in Uygur adults. In other studies, Frühbeck et al. reported that the adiponectin/leptin ratio is a good indicator of a dysfunctional adipose tissue that may be a useful estimator of obesity- and MS-associated cardiometabolic risk, allowing the identification of a higher number of subjects at risk (10,11). In our study, we did not observe any relationship between DII and adiponectin/leptin ratio after controlling for age, gender, and BMI. More studies are needed to confirm these observations in the future. In our study, we also did not observe DII as related to other inflammatory indices (CRP, IL-6, TNF-α) as other studies did (36,37). A study has reported that the dietary pattern has an important role in affecting circulating inflammatory markers in adults (38), but influencing factors involve all aspects, not just one. Staying up late, sedentary behavior and other lifestyle play a key role in regulating inflammation and health (39).
Obesity is also linked to dyslipidemia, caused primarily by insulin resistance and pro-inflammatory adipokines (40). In this study, we found that the concentration of HDL-C was negatively correlated with DII score after adjustment for age, gender and BMI in a linear regression model. The result was consistent with that by Neufcourt et al. (41), who demonstrated that higher DII scores were associated with higher TG and lower HDL-C. Abdollahzad et al. (42) also confirmed a correlation between DII score and lipid profile.
Our study has several strengths and limitations. To our knowledge, this is the first study to examine the relationship between DII and adipocyte-related factors in rural Uygurs. A pro-inflammatory diet, as indicated by a higher DII score, is associated with adipose tissue inflammation in Uygur adults and supports the hypothesis that diet may have a role in the development of obesity through inflammatory modulation mechanisms. As a cross-sectional study it also has some limitations. First, there were only 22 food parameters that were used to calculate DII in this study. A few food parameters were not available for calculating DII scores. For instance, coffee, thyme/oregano, rosemary, green/black tea, etc., are very scarcely or not at all consumed by this population. Second, the small sample size may have an impact on the interpretation of the results, so it is necessary to increase the sample size in different regions and populations to verify the usefulness of DII. To be sure, diet can modulate inflammation, but this does not mean that it is the only influencing factor. Finally, there are many other factors that will have certain effects — especially sedentary behavior and physical activity play a key role in regulating inflammation and health (39). In this study, participants with a high pro-inflammatory diet (third tertile) were more likely to be female, married, and less educated. The majority of overweight/obese people were female (68.20 %) because in the season when the study began, some young and middle-aged men went out to work, while some women stayed at home. Besides, we observed according to the tertiles of DII that higher educated people prefer to choose an anti-inflammatory diet, which is possibly related to their health literacy. Therefore, these results must be taken into account when explaining them. It is suggested that we should pay attention to these problems in our next research work.
CONCLUSIONS
In summary, our study showed that the DII score was negatively associated with HDL-C and ADPN, and positively associated with LEP concentration. These results support that diet has a certain influence on adipose tissue inflammation, and an anti-inflammatory diet is associated with a lower risk of developing obesity in Uygur adults. A healthy lifestyle (including choosing an anti-inflammatory diet) may help guide local people, and may also open up new areas of research.














