INTRODUCTION
Metabolic syndrome is the name for a group of risk factors that raises the risk for heart disease and other health problems (1). Metabolic syndrome may be diagnosed if a subject meets three or more of the following criteria: a) being overweight or having excess fat around the waist; b) high triglyceride levels and low levels of high-density lipoprotein cholesterol (HDL-c); c) high blood pressure (consistently 140/90 mmHg or higher); and d) inability to control blood sugar levels (insulin resistance) (2). The incidence of metabolic syndrome often parallels the incidence of obesity and type 2 diabetes, so the global prevalence of metabolic syndrome can be estimated to be about one-quarter of the world population (2).
Metabolic associated fatty liver disease (MAFLD), before named as Non-alcoholic fatty liver disease (NAFLD) is a liver disease associated with metabolic syndrome and all its risk factors, obesity, insulin resistance, type 2 diabetes mellitus, hypertension, and hyperlipidemia (3).
Heart-healthy lifestyle changes, including dietary changes, weight control, management of stress, physical activity, and quitting smoking, are the first line of treatment for metabolic syndrome. Statins, aspirin, and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers prescribed in evidence-based doses may be helpful as adjuncts to lifestyle changes in the treatment of metabolic syndrome, but they do not represent alternatives (4). Moreover, although agents are being tested in clinical trials for their ability to reverse the effects of fatty liver, the only proven treatments are weight loss and increased physical activity, which are hard to sustain (5). Among the dietary intervention measures and lifestyle changes contemplated by the latest consensus document for the management of this disease is the Mediterranean diet (MD) (evidence B1) (6).
S-adenosyl-L-methionine, N-acetylcysteine, thioctic acid, and pyridoxine (vitamin B6) are four powerful antioxidants. S-adenosyl-L-methionine donates methyl groups in essential protein, lipid, and nucleic acid methylation reactions (7). N-acetylcysteine has an antioxidant and protective action. It is a precursor of cysteine in the hepatic transsulfuration pathway, and it donates reducing groups and acts against oxidized free radicals (8). Thioctic acid is a short chain fatty acid that stimulates the glutamate-cysteine ligase enzyme that synthesizes glutathione from cysteine (9). Thioctic acid mediates the recovery of reduced glutathione from oxidized glutathione and, thanks to its thiol group, neutralizes radicals and recovers glutathione and other antioxidants, such as Q-10 and vitamins C and E (10). Pyridoxine is a water-soluble vitamin that must be replaced with the diet daily. It is a cofactor that induces the hepatic transsulfuration pathway by activating the cystathionine-β-synthase and cystathionine γ-lyase enzymes. This prevents the increase in methionine and contributes to the normal metabolism of homocysteine (11).
The main aim of this study was to evaluate the effect of supplementation with S-adenosyl-L-methionine + N-acetylcysteine + thioctic acid + vitamin B6 (MetioNac®) for 3 months on lipidic [total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), very low density lipoprotein cholesterol (VLDL-c)] and biochemical [glucose, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and γ-glutamyl transpeptidase (GGT)] parameters in subjects with metabolic syndrome and at risk of MAFLD. The reduction in body weight and the oxidative stress markers malondialdehyde (MDA) and superoxide dismutase (SOD) were also evaluated.
METHODOLOGY
DESIGN
This was a randomized pilot clinical trial carried out by the Nutrition and Digestive System Units of Hospital Ruber Internacional Paseo de la Habana (Grupo Quirón), with approval, on February 17, 2020, from the Research Ethics Committee with medicinal products of Grupo Hospitalario QuirónSalud-Catalunya. It complies with the guidelines indicated by Good Clinical Research Practices, the Biomedical Research Law 14/2007, and the Declaration of Helsinki and its subsequent revisions (Fortaleza, Brazil, 2013). The confidentiality of patient data was respected in accordance with the Organic Law 3/2018, of December 5, on the Protection of Personal Data and Guarantee of Digital Rights. Informed written consent was obtained from all participants, before the study procedures.
SUBJECTS
Men and women between the ages of 45 and 65 years, with metabolic syndrome, at risk of MAFLD (FIB-4 < 1.30), and with an indication for weight reduction were recruited. Metabolic syndrome was considered present when abdominal circumference was ≥ 94 cm in men and ≥ 80 cm in women and when two or more of the following criteria were also met: triglycerides ≥ 150 mg/dl or 1.7 mmol/l, HDL-c < 40 mg/dl in men and < 50 mg/dl in women, systolic pressure ≥ 130 and/or diastolic pressure ≥ 85 mmHg, and fasting glycemia ≥ 100 mg/l (5.6 mmol/l) or type II diabetes.
Patients with an indication for bariatric surgery, hepatitis C or B virus infection, uncontrolled type I or type II diabetes (>127 mg/dl or 6.5 % HbAc1), fibrosis stage F2 or higher, or diagnosis of a chronic disease other than type 2 diabetes, hypertriglyceridemia, or hypertension were excluded. Also excluded were (a) patients who received chronic pharmacological treatment or had any other pathology requiring medical intervention during the duration of the study, except for the purposes of glycemic control or reduction of triglyceride levels or blood pressure; (b) patients who were receiving drug treatment for fibrosis; and (c) patients who failed to express an intention to abstain from alcohol or to adhere to the dietary and exercise recommendations. Pregnant and lactating patients could not participate.
After assessment at the baseline visit, patients meeting all the inclusion and exclusion criteria were randomly divided into two groups. Group A (control) followed a semipersonalized MD for weight reduction, according to the recommendations of the Spanish Society for the Study of Obesity (SEEDO). Group B, in addition to following the MD, took three capsules of MetioNac® supplement per day, two in the morning and one in the evening. Patients returned to the clinic for a follow-up visit at week 4 and at the end of the study. The intervention lasted for 3 months.
SAMPLE SIZE CALCULATIONS
The basal glucose levels (with a reference range of 75-106 mg/dl with a variance of 31 mg/dl) was expected to improve by 22-25 mg/dl. A dropout rate of 5 % was expected. The calculated sample size per group needed to be 19, which was rounded up to ≥ 20, to achieve a power of ≥ 75 % with α = 0.05 and a confidence interval of 95 %. Randomization was carried out using a random number table and an assignment group table.
FOOD SUPPLEMENT
MetioNac® is a food supplement that combines S-adenosyl-L-methionine (200 mg), N-acetylcysteine (100 mg), thioctic acid (75 mg), and vitamin B6 (0.65 mg). MetioNac® was formulated to help the synthesis of glutathione from intermediate metabolites that intervene in the metabolic pathways of hepatic methylation and transsulfuration. MetioNac® was dispensed in tablets formulated with LUBRITAB®, a patented extended-release excipient for direct compression formulas.
DIET
In addition to the administration of MetioNac®, participants were given a semipersonalized MD. This diet was adjusted to the intolerances and allergies of each person. Energy expenditure was calculated using the Harris-Benedict equation (12) to determine the caloric intake. As recommended by the Spanish Society for the Study of Obesity (SEEDO), diets with a reduction of 500–1000 kcal/day were provided for weight loss (13). Four diets were designed, one for each week, and different interchangeable options were given in each diet.
DATA COLLECTION
Liver profile biomarkers, including AST, ALT, alkaline phosphatase, GGT, and albumin, were measured. Metabolic response was monitored by abdominal circumference, HDL-c, LDL-c, and VLDL-c levels, triglycerides, blood pressure, and fasting glucose. Oxidative stress markers such as MDA and SOD were measured. The NAFLD fibrosis score (NFS) was also recorded. The NFS (14) is a noninvasive scoring system based on several laboratory tests that help to estimate the amount of scarring in the liver. The NFS is based on the following formula: -1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet (×109/l) – 0.66 × albumin (g/dl). The NFS results are classified as follows: NFS < -1.455 = F0-F2 (no, mild, or moderate fibrosis); NFS -1.455 to 0.675 = indeterminate score; and NFS > 0.675 = F3-F4 (severe fibrosis or cirrhosis). Weight and body composition were measured by bioimpedance with a Tanita BP601 in accordance with standardized recommendations. The differences between the aforementioned biomarkers and indices were measured at the beginning and at the end of the intervention.
DATA ANALYSIS
SPSS 27.0 software was used to analyze the results. All data acquired, including age, sex, anthropometric measurements, and clinical markers, were descriptively analyzed. Means and standard deviations were calculated for quantitative variables and frequencies for qualitative variables. The existence of statistically significant differences (p < 0.05) between the qualitative variables was established after the completion of the chi-square test and/or ANOVA.
RESULTS
DESCRIPTION OF THE STUDY POPULATION
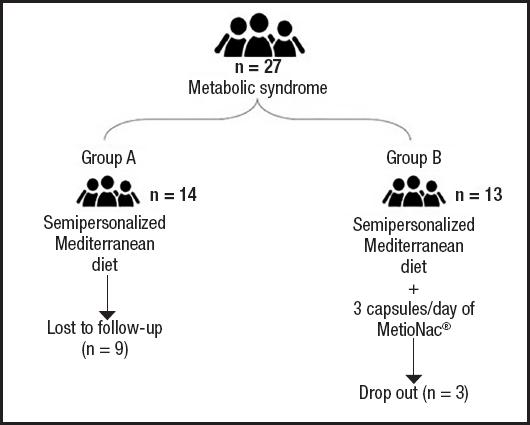
Between March 2021 and June 2021, 27 patients with metabolic syndrome and at risk of NAFLD were randomly assigned to receive either just a semipersonalized MD (control group) or MetioNac® in addition to a semipersonalized MD (MetioNac® group). In the control arm, five patients completed the study, with nine lost to follow-up. In the MetioNac® arm, ten patients completed the study, with three dropping out (Fig. 1).
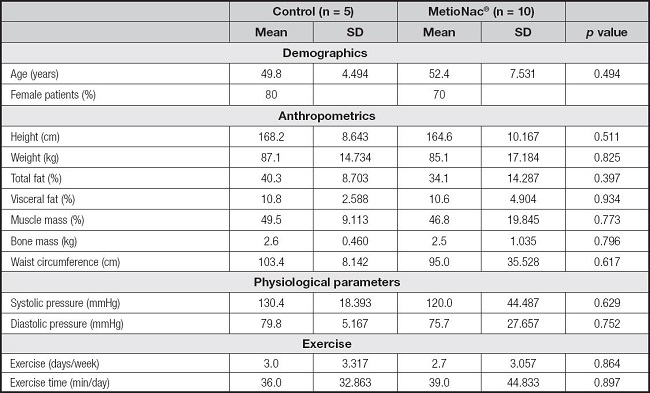
Seventy-three percent of the study population were women. The two groups, control and MetioNac®, had similar baseline characteristics (p > 0.05) (Table I).
EFFECT OF METIONAC® ON BODY WEIGHT AND THE LIPIDIC PROFILE
In this study, weight loss was programmed through an MD. The control group displayed a significant reduction (p = 0.026) in weight (-4.1 ± 2.7 kg) after following the MD diet; the MetioNac® group also succeeded in achieving weight loss (-2.9 ± 6.569 kg) but the reduction did not reach significance (p > 0.05) (Table II).
Table II. Baseline and final biochemical characteristics and NAFLD fibrosis score (NFS) and their paired differences after treatment.

AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT; ?-glutamyl transpeptidase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low density lipoprotein; NAFLD: nonalcoholic fatty liver disease; MDA: malondialdehyde; SOD: superoxide dismutase.
*Statistically significant (p < 0.05).
Regarding lipidic profile, patients who were taking the MetioNac® supplement showed reductions in total cholesterol (TC) and LDL-c levels from 206.6 ± 37.3 to 192.4 ± 35.2 mg/dl and from 127.0 ± 26.3 to 119.0 ± 35.2 mg/dl, respectively (Fig. 2). In contrast, control subjects displayed increased TC and LDL-c levels (Table II). Moreover, patients taking MetioNac® had a significant reduction (p = 0.048) in VLDL-c (Table II; Fig. 2) and TG levels (p = 0.043) (Fig. 2). In addition, in MetioNac® group levels of HDL-c increased by 1.7 ± 9.6 mg/dl after treatment.
EFFECT OF METIONAC® ON SERUM GLUCOSE, AST, ALT, AND GGT LEVELS
Secondary outcome measures were changes in serum glucose, albumin, AST, ALT, and GGT levels. In the present study, supplementation with MetioNac® helped reduce the serum glucose levels, while in the control group, glucose levels continued to rise (Table II; Fig. 2).
We also observed decreased levels of AST and ALT after the intervention with MetioNac®, although the reductions were higher in the control group (Table II). Regarding GGT levels, only the control group benefited from a decrease (71.4 ± 84.9 to 52.2 ± 50.5 U/l) (Table II).
EFFECT OF METIONAC® ON MDA AND SOD LEVELS
The oxidative stress markers were also evaluated. We did not observe that either supplementation with MetioNac® or the MD lowered the levels of MDA or SOD (Table II).
EFFECT OF METIONAC® ON THE NAFLD FIBROSIS SCORE (NFS)
Changes in the NFS (Table II) were also recorded. At the end of the follow-up, we classified the patients into three subgroups according to the pattern of progression of liver fibrosis by comparing the NFS at baseline to the NFS at the end of the follow-up period. Most patients were in the progressive fibrosis (60 %) group, while 33.3 % were in the regressive fibrosis group and 6.7 % remained stable.
DISCUSSION
The first-line approach to control metabolic syndrome is weight control and exercise. The MD with components such as fish, nuts, fruits, olive oil, whole grains, and vegetables was declared by the United Nations Educational, Scientific and Cultural Organization (UNESCO) to be a diet with the ability to preserve the state of health and improve longevity (15). The MD pattern have been found to be inversely related to the body mass index (16). The MD has also been proposed as an effective diet to lower the fat around the mid-section (17). In this study, in which weight loss was programmed through an MD, the control group displayed a significant reduction in weight (-4.1 ± 2.7 kg) whereas the MetioNac® group, although also succeeded in achieving weight loss (-2.9 ± 6.569 kg), did not reach significance.
Metabolic syndrome is characterized by elevated triglycerides and reduced HDL-c (2), and although individuals with the metabolic syndrome often have average levels of LDL-c, they may have qualitative abnormalities, such as small dense LDL particles (18). Since NAFLD is a hepatic manifestation of metabolic syndrome, excessive cholesterol deposition in the liver is presumed to be a risk factor for disease progression (19). The lifestyle changes recommended by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) for control of dyslipidemia (i.e., elevated levels of triglycerides and decreased levels of HDL-C) in patients with metabolic syndrome include (a) reduced intake of saturated fats and dietary cholesterol, (b) intake of dietary options to enhance lowering of LDL-c, (c) weight control, and (d) increased physical activity (20). A study by Babio et al. (17) suggested that the MD may reverse metabolic syndrome, improve the conglomeration of markers that includes high cholesterol, triglycerides, and blood pressure, and reduce insulin resistance. In our study, patients who were taking the MetioNac® supplement showed reductions in total cholesterol (TC) and LDL-c levels from 206.6 ± 37.3 to 192.4 ± 35.2 mg/dl and from 127.0 ± 26.3 to 119.0 ± 35.2 mg/dl, respectively. In contrast, control subjects displayed increased TC and LDL-c levels. Moreover, patients taking MetioNac® had a significant reduction in VLDL-c levels. Excess TG accumulation in the arteries increases the risk of stroke, heart attack, and heart disease (21), while TG accumulation in the liver is a hallmark of MAFLD (22). Since TG is a potential source of oxidative stress, it has been considered to be a “bad fat” (22). We found that MetioNac® was able to reduce TG levels significantly, meaning it may represent a good option to tackle the latter issue. Summaries of the lipid profile in metabolic syndrome also show depleted plasma HDL-c (23). This was also observed in our MetioNac® group, in which levels of HDL-c increased by 1.7 ± 9.6 mg/dl after treatment. In this context, Tortosa et al. (24) previously concluded that HDL-c levels were higher among participants with metabolic syndrome who better adhered to the Mediterranean food pattern. Nevertheless, lipid profile after treatment has to be verified simultaneously because the association between lipid profile and treatment efficacy has not yet been determined.
On the other hand, there are several clinical determinants of progression of fibrosis in MAFLD. The presence of insulin resistance is one of the major predictors of fibrosis progression (25) as well as being an indicator for metabolic syndrome (2). Systemic insulin resistance is characterized by the inability of insulin to reduce blood glucose levels appropriately (26). As the production rates of glucose are highly aberrant in hepatocytes in the presence of a high insulin level, this is characterized as a sign of hepatic insulin resistance (27). The results of the present study showed that supplementation with MetioNac® helped reduce the serum glucose levels, while in the control group, glucose levels continued to rise. Greater adherence to the MD was associated with a lower degree of insulin resistance (28), which may explain the reductions in glucose levels in our study. In a randomized, cross-over intervention trial, Ryan et al. (29) showed that, compared with a low-fat high-carbohydrate diet, MD improved insulin sensitivity and hepatic steatosis in patients with biopsy-proven MAFLD in the absence of weight loss.
Other clinical determinants of MAFLD diagnosis and fibrosis progression rate are ALT, AST, and GGT levels above the upper limits of normal (30). We observed higher reduction of AST and ALT levels in the control group and, regarding GGT levels, only the control group benefited from a decrease (71.4 ± 84.9 to 52.2 ± 50.5 U/l). In a study by Mansour-Ghanaei et al. (31), the mean values of ALT, AST, and GGT in the NAFLD group were higher than those in the non-MAFLD group. The authors also observed that, when they compared the changes in biochemical parameters with different degrees of MAFLD, there was a relationship between GGT (p = 0.004), ALT (p = 0.007), and AST (p < 0.001) and the severity of fatty liver.
During the process of lipid peroxidation, a wide range of preinflammatory products are produced that result in progression of the disease. One of these by-products is MDA, which is a common marker for oxidative stress (32). In a study by Moreto et al. (33), subjects with higher plasma MDA showed a higher prevalence of MetS and higher values of waist circumference, glucose, triglycerides, and γ-glutamyl transferase. An increase in serum oxidative markers (e.g., MDA) paralleled by a decrease in the activity of antioxidants has also been observed in patients with MAFLD (34). Varma et al. (35) demonstrated plasma MDA levels to be significantly increased in diabetic or obese MAFLD patients as compared with healthy controls. Interestingly, Kumar et al. (36) also demonstrated how patients with MAFLD have significantly higher levels of MDA and other oxidative markers in comparison to chronic viral hepatitis patients. According to Kani et al. (37), dietary intervention followed by weight reduction leads to a reduction in serum MDA. Specifically, the Dietary Approaches to Stop Hypertension (DASH) diet, which is abundant in antioxidants and is designed to be rich in fruits, vegetables, and whole grains, led to a reduction in the serum levels of inflammatory markers, including MDA (38). However, we did not observe that either supplementation with MetioNac® or the MD, which is similar in concept to the DASH diet, lowered the levels of MDA. SOD, another plasma oxidative stress-related parameter, followed the same trend. When other dietary compounds such as vitamins E and C were tested by Zelber-Sagi et al. (39) in the search for an association between these and MDA levels, the authors found an inverse association between dietary vitamin E intake and serum MDA level.
Finally, at the end of the follow-up, we classified the patients into three subgroups according to the pattern of progression of liver fibrosis. Most patients were in the progressive fibrosis (60 %) group, while 33.3 % were in the regressive fibrosis group and 6.7 % remained stable. Treeprasertsuk et al. (39) classified MAFLD patients similarly and observed that most patients were in the stable fibrosis (60 %) and progressive fibrosis (37 %) groups, with only 3 % in the regressive fibrosis group, after the use of statins and metformin during the follow-up period. The NFS uses two diagnostic cutoffs, a low cutoff score (-1.455) to exclude advanced fibrosis (negative predictive value 88 %-93 %) and a high cutoff score (0.676) to diagnose advanced fibrosis (positive predictive value 82 %-90 %) (14), leaving one-third of patients in a “gray zone” where liver biopsy is still required. In our study, all patients remained below the low cutoff score. However, it is worth mentioning that the MD has shown an inverse relationship with MAFLD prevalence (40), that it reduces liver steatosis (29), and that patients in the present study could have benefited from it.
CONCLUSIONS
Early recognition and proper management of metabolic syndrome and MAFLD are of major importance. Various indicators such as lipid profile, AST, ALT, GGT, plasma glucose, and fasting insulin level play a significant role in metabolic syndrome and MAFLD. These indicators assist in understanding the severity and prognosis of the diseases, and can offer a basis for early intervention. However, none of the currently available biomarkers have sufficient accuracy to enable diagnosis, which is why predictive scores play an important role in providing a cutoff capable of distinguishing between absence of fibrosis and presence of advanced fibrosis in the context of MAFLD.
Given the complex physiopathology of metabolic syndrome and MAFLD, it is unlikely that one drug by itself will deliver significant clinical outcomes. On the other hand, combinations of components like MetioNac® (S-adenosyl-L-methionine, N-acetylcysteine, thioctic acid, and vitamin B6) with different targets could perhaps aid in improving metabolic syndrome and, in consequence, risk of MAFLD. The ultimate goal of this approach should be to establish an opportune treatment that decreases or halts disease progression and improves prognosis.
LIMITATIONS
The promoter and CRO are aware of the pilot and exploratory nature of the study. The reason for the high dropout rate was the COVID-19 pandemic as the study was conducted in parallel with the 3 months lockdown followed by intermittent mobility restrictions for 1 year. Other relevant limitations were the sample size, the lack of measurement of an abdominal ultrasound or FibroScan to confirm the presence of liver fibrosis and assess the potential improvement of treatment, instead of measuring and evaluating MAFLD risk biomarkers.