INTRODUCTION
Esophageal cancer causes more than 500,000 cancer deaths each year, ranking sixth among all cancer-related deaths (1). The five-year overall survival rate for patients with esophageal cancer worldwide ranges from 15 % to 25 % (2), and risk factors for esophageal cancer include alcohol consumption, smoking, lack of fruits and vegetables, obesity, and gastroesophageal reflux disease (3). Esophagectomy is still the main treatment method for esophageal cancer, but severe trauma and postoperative complications, such as esophageal anastomotic leakage, gastroesophageal reflux, and severe infection of esophagectomy may impair the patients’ quality of life (4).
Enteral immunonutrition (EIN), which can reduce the production of inflammatory mediators and regulate eicosanoid synthesis, is an enteral formula containing arginine, glutamine, omega-3 fatty acids and nucleotides (5-7). Immunonutrition has been introduced and proposed to improve the nutritional status of the body, enhance the response function of immune cells, regulate the production and release of cytokine and reduce inflammatory markers for surgical patients (8-10). However, the effect of EIN in EC patients remains unclear. Wang et al. (11) have conducted a preliminary analysis of EIN treatment after esophageal cancer and found that EIN did not reduce the incidence of postoperative complications in EC patients. Based on this study, we included ten RTCs and updated the meta-analysis on the relationship between inflammatory markers or postoperative complications with EIN after esophageal cancer surgery.
MATERIALS AND METHODS
SELECTION CRITERIA
The inclusion criteria were as follows: a) published randomized controlled trials (RCTs) of immunonutritional support in EC patients with complete data and no language restrictions; b) subjects: all EC patients who received preoperative and/or postoperative immunonutrition support, and the duration of nutritional support was not limited; c) intervention measures: the experimental group was given immune nutritional support, and the control group was given routine nutritional support; d) outcome measures: the main outcome measures were the incidence of pneumonia, anastomotic leakage, surgical site infection, intra-abdominal abscess, septicemia, urinary tract infection, acute respiratory distress syndrome (ARDS), in-hospital mortality, C-reactive protein (CRP) of postoperative day (POD) 1, POD 3 and POD 7, and IL-6 of POD 1. The exclusion criteria were as follows: a) non-randomized controlled trials, such as reviews, systematic reviews, case reports, disease syndrome definition, etc.; b) non-clinical experiments, such as animal, cell experiments, etc.; and c) incomplete or duplicate information; and d) duplicate literature.
SEARCH STRATEGY
PubMed, Embase, and the Cochrane library were systematically searched from inception to April 2022, with the following keywords: (“oesophagus resection” or “esophagectomy” or “resection of esophagus” or “oesophagectomy” or “esophagus cancer” or “esophageal cancer” or “esophageal squamous cell carcinoma” or “esophageal carcinomas” or “oesophageal cancer” or “oesophageal carcinoma” or “carcinoma of the esophagus” or “carcinoma of esophagus” or “esophageal carcinoma” “esophagus carcinoma”) and (“immunonutrition” or “immune-enhancing” or “immune-enhanced” or “immune-modulating”). No limitation was enhanced. To include additional eligible studies, the reference lists of retrieved studies and relevant reviews were also hand-searched and the process above was performed repeatedly until no further article was identified. Conference abstracts meeting the inclusion criteria were also included.
DATA EXTRACTION AND QUALITY ASSESSMENT
Two researchers independently extracted the following information of RCTs according to predefined selection criteria: name of first author, publication year, sample size, baseline characteristics of patients, EIN formula and usage, control, study design, pneumonia, anastomotic leakage, surgical site infection, intra-abdominal abscess, septicemia, urinary tract infection, ARDS, in-hospital mortality, CRP of POD 1, POD 3 and POD 7, and IL-6 of POD 1. The quality assessments of eligible studies were performed using the Cochrane Collaboration's tool published in the Cochrane Handbook (version 5.3).
DATA ANALYSIS
Meta-statistical analysis was performed using RevMan 5.3 software. Input raw data and perform data transformation. Mean differences (MDs) with 95 % confidence intervals (CIs) for continuous outcomes, and risk ratios (RRs) with 95 % CIs for dichotomous outcomes were used to estimate the pooled effects. Each effect size is provided with 95 % CI. If p ≥ 0.05, I2 ≤ 50 %, a fixed-effect model was used for analysis; if p < 0.05, I2 > 50 %, it was considered that there was significant heterogeneity among studies, and subgroup analysis was performed or omitting one study at a time. If the heterogeneity cannot be eliminated, the random effects model is used to combine the effect sizes.
RESULTS
LITERATURE SEARCH, STUDY CHARACTERISTICS AND QUALITY ASSESSMENT
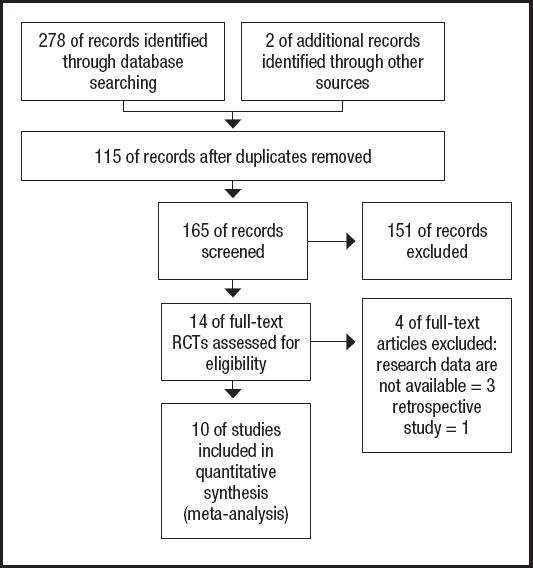
The PRISMA flow diagram for the selection process and detailed identification was presented in figure 1. Two hundred and eighty publications were identified through the initial search of databases. After screening, ten RCTs (12-21) were included in the meta-analysis. And the basic characteristics of the ten publications included in this meta-analysis are presented in table I. These ten articles were published from 2007 to 2020, and the sample size included in these articles ranged from 29 to 272 with a total of 1,052, 573 of which received EIN before and/or after surgery and 479 received perioperative enteral nutrition (EN). Two studies included 112 patients who did not receive EN before esophagectomy and started EIN or EN after surgery. One study included 69 patients who received preoperative EIN without postoperative EIN, 68 patients who received postoperative EIN without preoperative EIN, and 77 patients who received both preoperative and postoperative EIN. And seven studies included 668 patients who received postoperative and postoperative EIN or EN.
Table I. The basic characteristics of involved trials (EN/EIN).
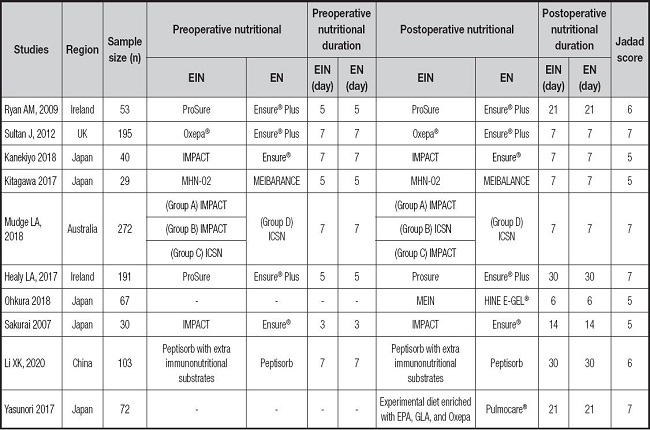
EIN: enteral immunonutrition; EN: enteral nutrition; EPA: eicosapentaenoic acid; GLA: γ-linolenic acid.
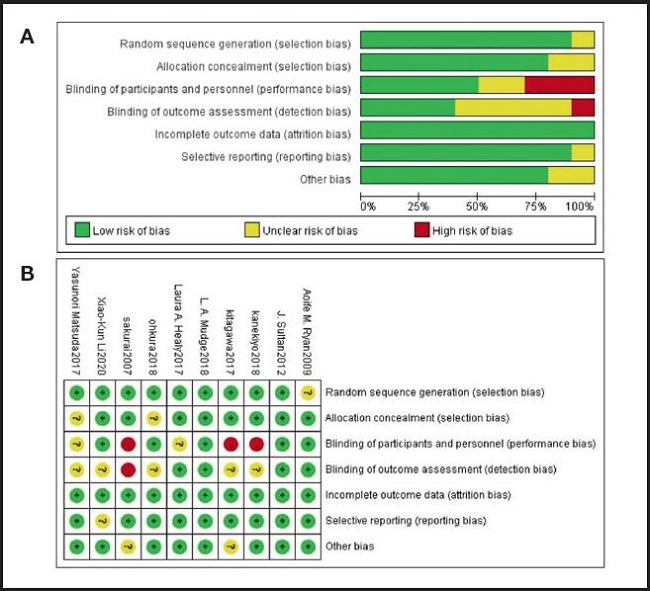
The quality of each study was evaluated, most of the studies were high-quality RCTs, and their quality assessment is listed in figure 2. The modified Jadad scale was used to evaluate the methodological quality of each RCT included in this meta-analysis. All ten studies were considered to be high-quality ones according to quality assessment.
INFECTION-RELATED COMPLICATIONS AND HEMATOLOGICAL INDICATORS
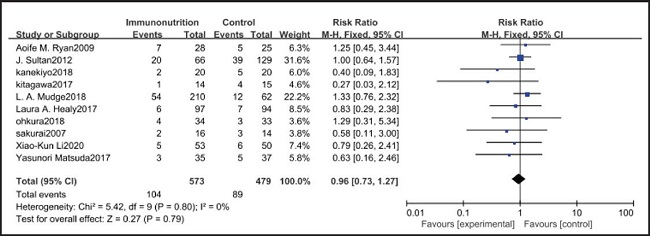
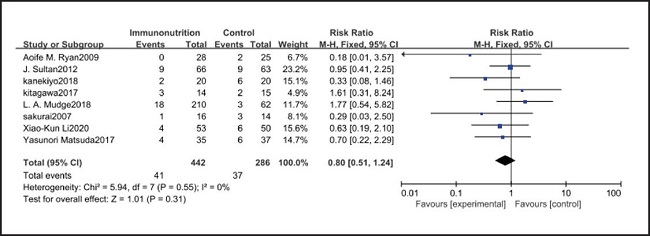
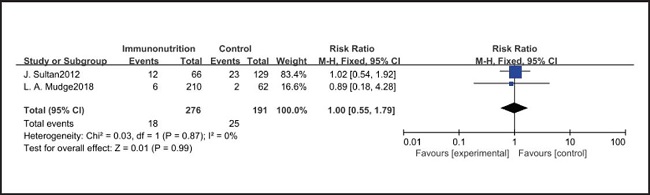
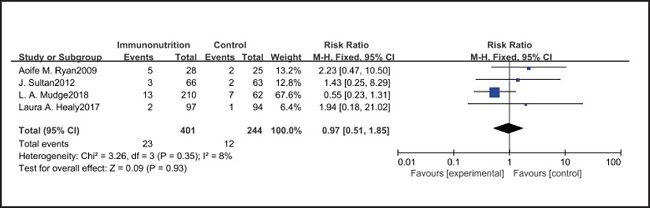
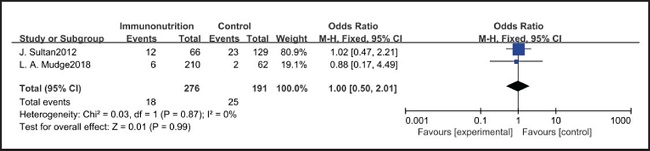
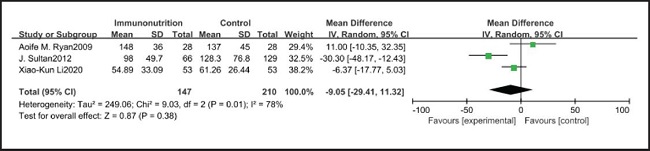
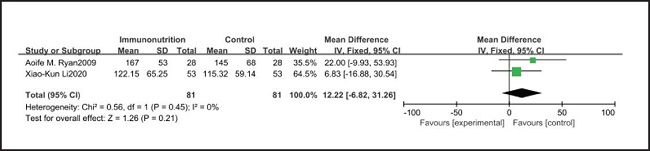
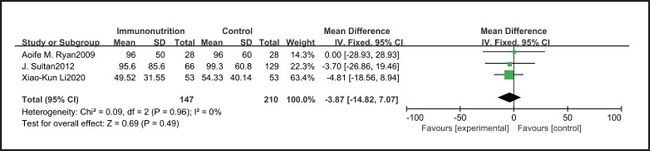
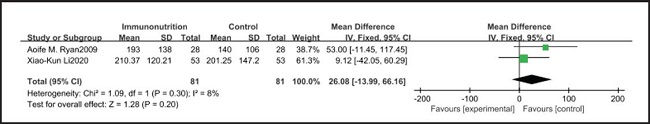
All ten included studies reported the incidence of pulmonary infection, but there was no significant difference in the incidence of pneumonia between the EIN and EN group (RR = 0.96, CI: 0.73-1.27, p = 0.79) (Fig. 3). Eight of the ten included studies reported the incidence of wound infection, but there was no significant difference between the EIN and EN group (RR = 0.80, CI: 0.51-1.24, p = 0.31) (Fig. 4). Two of the ten included studies reported the incidence of intra-abdominal abscess, but there was no significant difference between the EIN and EN group (RR = 1.00, CI: 0.55-1.79, p = 0.99) (Fig. 5). Four of the ten included studies reported the incidence of septicemia, but the incidence of septicemia was not significantly different between the EIN and EN group (RR = 0.97, CI: 0.51-1.85, p = 0.93) (Fig. 6). Two of the ten included studies reported the incidence of urinary tract infection, but there was no significant difference between the EIN and EN group (RR = 1.00, CI: 0.50-2.01, p = 0.99) (Fig. 7). All eligible studies provided the incidence of infection complications, which included CRP of POD 1 in three studies, CRP of POD 3 in two studies, CRP of POD 7 in three studies, and IL-6 of POD 1 in two studies. No significant difference was observed between the two groups in CRP of POD 1 (MD = -9.05, CI: -29.41-11.32, p = 0.38) (Fig. 8), CRP of POD 3 (MD = 12.22, CI: -6.82-31.26, p = 0.21) (Fig. 9), CRP of POD 7 (MD = -3.87, CI: -14.82-7.07, p = 0.49) (Fig. 10), or IL-6 of POD 1 (MD = 26.08, CI: -13.99-66.16, p = 0.20) (Fig. 11).
DRUG SAFETY EVALUATION
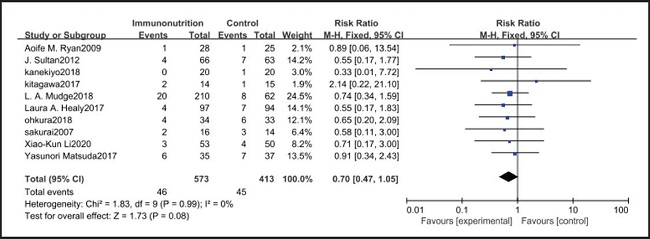
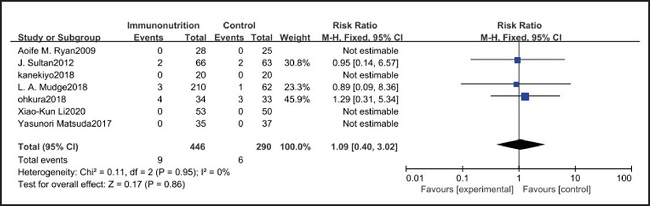
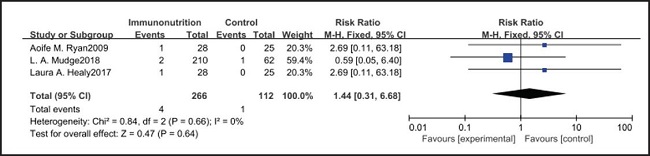
All ten included studies reported the incidence of anastomotic leakage, but there was no significant difference between the EIN and EN group (RR = 0.70, CI: 0.47-1.05, p = 0.08) (Fig. 12). Seven studies reported the in-hospital mortality rate, but there was no significant difference between the EIN and EN group (RR = 1.09, CI: 0.40-3.02, p = 0.86) (Fig. 13). And three studies reported the incidence of ARDS, but there was no significant difference between the EIN and EN group (RR = 1.44, CI: 0.31-6.68, p = 0.64) (Fig. 14).
DISCUSSION
Progressive dysphagia, first solid and then liquid, is a typical symptom of esophageal cancer. Therefore, most patients with esophageal cancer face a huge risk of malnutrition (22), and the median weight loss is the highest reported in esophageal cancer patients compared to patients with other malignancies (23). More studies show high risk of malnutrition and preoperative weight loss are associated with worse outcomes (24-29).
A meta-analysis aimed to evaluate the impact of EIN on postoperative infection and mortality in patients undergoing cancer surgery and indicated that EIN can reduce overall infectious complications and surgical-site infection (30). Yu et al. reported that immunonutrition did not reduce sepsis or all-cause mortality in cancer patients treated with surgery, but subgroup analyses revealed that immunonutrition for > 5 days and for ≤ 7 days reduced the rate of respiratory tract infection and the incidence of wound infection (31). However, another meta-analysis showed that there was no significant difference in infectious complications between immunonutritional support and traditional nutritional support after head and neck cancer surgery (32). An increasing number of controlled studies have focused on EIN and esophagectomy, but have not yet achieved ideal results.
Pulmonary infection is one of the most common complications after esophagectomy. It can be caused by many factors, such as surgical trauma, postoperative immunosuppression, sputum accumulation and disconnection of bronchial nerve. The current meta-analysis shows that there is no significant difference in the incidence of pulmonary infection between the EIN group and the EN group (RR = 0.96, CI: 0.73-1.27, p = 0.79). In our opinion, the pain of the surgical incision in esophagectomy inhibits the patient's voluntary cough, and expectoration may have a more significant impact on pulmonary infection, but all studies have not shown the patient's pain score and postoperative analgesia regimen. Consistent with our view, Yin et al. (33) reported that compared with thoracoscopic esophagectomy, transcervical and transhiatal esophagectomy has lower pain score and less pulmonary infections. In addition, Sluis et al. (34) findings show that robot-assisted minimally invasive thoracolaparoscopic esophagectomy has lower mean postoperative pain and lower percentage of pulmonary complications than open transthoracic esophagectomy. On the other hand, the current meta-analysis showed that there was no significant difference in wound infection (RR = 0.80, CI: 0.51-1.24, p = 0.31), septicemia (RR = 0.97, CI: 0.51-1.85, p = 0.93), urethral infection (RR = 1.00, CI: 0.50-2.01, p = 0.99), intra-abdominal abscess (RR = 1.00, CI: 0.55-1.79, p = 0.99) and ARDS (RR = 1.44, CI: 0.31-6.68, p = 0.64) between the EIN group and the EN group. In the general view, the above-mentioned infectious complications may be related to deep venous catheterization, intraoperative aseptic management, and postoperative incision dressing change. Therefore, EIN and EN did not show significant differences in these complications. This meta-analysis showed that although the incidence of anastomotic leakage was lower in the EIN group, it did not show a significant difference compared with the EN group (RR = 0.70, CI: 0.47-1.05, p = 0.08). We deem that the occurrence of anastomotic leakage may be related to the blood supply and the tension of the anastomosis, and the postoperative inflammatory state may be a secondary factor, and only single-factor intervention cannot reduce the occurrence of anastomotic leakage.
Studies have shown that EIN can reduce the inflammatory response in severe patients with Covid-19, severe acute pancreatitis, major abdominal surgery and so on (35-37). But results of this meta-analysis showed that there was no significant difference in CRP of POD 1 (MD = -9.05, CI: -29.41-11.32, p = 0.38), POD 3 (MD = 12.22, CI: -6.82-31.26, p = 0.21), POD 7 (MD = -3.87, CI: -14.82-7.07, p = 0.49) and IL-6 of POD 1 (MD = 26.08, CI: -13.99-66.16, p = 0.20) after esophagectomy between the EIN group and the EN group.
However, the inflammatory factors selected in the included studies may not fully reflect the inflammatory state of patients, therefore, more inflammatory indicators such as procalcitonin, ESR and leukocyte count need to be measured to evaluate the relationship between the body's inflammatory state and EIN. On the other hand, the included studies did not show the administration of antibiotics after operation, and antibiotics may have a more significant inhibitory effect on inflammatory response than EIN. However, there was no significant difference in the in-hospital mortality (RR = 1.09, CI: 0.40-3.02, p = 0.86) and incidence of ARDS (RR = 1.44, CI: 0.31-6.68, p = 0.64) between EN and EIN, which at least suggested that EIN was a safe treatment.