My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Anales de Psicología
On-line version ISSN 1695-2294Print version ISSN 0212-9728
Anal. Psicol. vol.30 n.2 Murcia May. 2014
https://dx.doi.org/10.6018/analesps.30.2.152921
Short polymorphism of the serotonin transporter (5-HTTLPR) gene and its association with the cortisol stress response: a meta-analysis
Polimorfismo corto del gen del transportador de la serotonina (5-HTTLPR) y su asociación con la respuesta al estrés mediada por cortisol: un meta-análisis
Emiliano Agüero-Tejado
University Hospital of Torrejón de Ardoz (Spain)
ABSTRACT
Several studies have been conducted to analyze the role of short polymorphism of serotonin transporter (5-HTTLPR) in response to psy-chosocial stress mediated by cortisol, having found inconsistent results. In order to synthesize and analyze available information about the role of short polymorphisms of 5-HTTLPR gen in the stress response, this meta-analysis has been carried out. A meta-analysis of studies published until November 2011 without language restrictions, which analyzed the effect of the short polymorphisms of 5-HTTLPR in the cortisol response during a stress-inducing protocol was conducted. Published F or p genotype values were collected as estimators of the difference between groups as well as the sign of this difference for analysis.
The global meta-analysis showed no significant association between the presence of the short allele and a different response to psychosocial stress. After comparing the subgroups of studies where participants underwent an emotionally neutral intervention versus an emotionally negative intervention, significant differences were found. The subgroup of studies where patients underwent an emotionally negative intervention showed a significantly greater cortisol release by those individuals carrying the short allele. This association was not seen in the neutral subgroup.
Key words: Serotonin transporter; gene; 5-HTTLPR; cortisol; hydrocortisone; stress; meta-analysis.
RESUMEN
Varios estudios han sido llevados a cabo para analizar el papel del polimorfismo corto del transportador de la serotonina (5-HTTLPR) en la respuesta al estrés psicosocial mediado por cortisol, habiéndose encontrado resultados contradictorios.
Con el fin de sintetizar y analizar la información disponible acerca del papel del polimorfismo corto del gen 5-HTTLPR en la respuesta al estrés se ha realizado este meta-análisis. Se realizó un meta-análisis de estudios publicados hasta noviembre de 2011 sin restricción de idioma, donde se analizaba el efecto del polimorfismo corto del 5-HTTLPR en la respuesta de cortisol durante un protocolo inductor de estrés. Se recogieron los valores F o p genotipo publicados como estimadores de la diferencia entre grupos, así como el signo de esta diferencia para su análisis.
El meta-análisis global no mostró asociación significativa entre la presencia del alelo corto y una diferente respuesta al estrés psicosocial. Tras comparar los subgrupos de estudios donde sometía a los participantes a una intervención emocionalmente neutral frente a una intervención emocionalmente negativa, se encontraron diferencias significativas. En el subgrupo de estudios donde los pacientes fueron sometidos durante la intervención a un ambiente emocionalmente negativo, se observó una significativamente mayor liberación de cortisol por parte de aquellos sujetos portadores del alelo corto. Esta asociación no se observó en el subgrupo neutral.
Palabras clave: Transportador de serotonina; gen; 5-HTTLPR; cortisol; hidrocortisona; estrés; meta-análisis.
Introduction
Stress is related to a variety of problems ranging from alterations in the immune system (Ho, Neo, Chua, Cheak & Mak, 2010; Jeckel et al., 2010), which has been observed as a greater incidence of influenza processes, herpesvirus-type recurrences (Ashcraft, Hunzeker & Bonneau, 2008; Cohen, Tyrrell & Smith, 1991) or a greater susceptibility to infections, to the appearance of heart diseases (Figueredo, 2009) and the development of mental diseases.
Since Post presented the kindling theory (1992), there has been a discussion but currently there is general acceptance about the role of stress in relation with the development of mental diseases (Monroe & Harkness, 2005). Nevertheless, not all individuals who face high stress levels suffer from these related diseases. On the contrary, individuals with milder stress levels suffer from these diseases and we can infer from this that there may be a factor or factors that make individuals more or less resistant to stress.
Stress plays a necessary role in our body as it prepares it to respond to threatening situations. In this preparation, an area of the brain called Hypothalamic-Pituitary-Adrenal axis is involved (Johnson, Kamilaris, Chrousos & Gold, 1992). This area is activated by neurotransmitters. Among these neurotransmitters in charge of activating the Hypothalamic-Pituitary-Adrenal axis, there is the serotonin transporter which has certainly been the most studied neurotransmitter. Its gen (SLCóA4) has two alleles; the short allele is related to a lower transcriptional activity and a reduction of the efficiency in the reuptake of serotonin, whereas the long allele carriers (L) have a better or quicker response to the reuptake of serotonin (Silva et al., 2010). Recently, it has been observed that the gen of serotonin transporter is a locus with three alleles named La, Lg and S, where Lg and S alleles could be compared regarding their low expressions levels as compared with La (Hu et al., 2006; Kraft, Slager, McGrath & Hamilton, 2005).
Studies carried out on twins have confirmed the importance of gene action in the response to stress (Steptoe, Van Jaarsveld, Semmler, Plomin & Wardle, 2009). Besides the serotonin transporter gene, there is evidence which indicates that stress perception by the individual may involve a complex genetic interaction, as there is information about the relation between the BDNF gene Val/Met polymorphism and sensitivity to stress (Alexander et al., 2010; Shalev et al., 2009). There is also a different response to stress pattern depending on polymorphism of the dopamine receptor D4 (Armbruster & Mueller, Moser, Lesch, Brocke & Kirschbaum, 2009) and it may even change due to epigenetic variations (Edelman et al., 2011). However, it was in studies on 5-HTTLPR gene where, a meta-analysis showed the association between a gene and the reactivity of a related brain zone, such as the amygdala, in a stressful situation (Munafo, Brown & Hariri, 2008). Or the gene-environment interaction between 5-HTTLPR gene and vital stressful events as predictors of depression, pathology related to stress (Karg, Burmeister, Schedden & Sen, 2011). A possible explanation might be the different stress sensitivity given by certain genes and the result in higher or lower cortisol release, caused by a different activation degree of the hypothalamic-pituitary-adrenal axis.
It is widely known that corticotropin-releasing hormone (CRH) secretion by the hypothalamus after a stressful situation triggers secretion from the hypophysis of the adreno-corticotropic hormone (ACTH). This hormone is transported through the blood to the adrenal cortex, where it triggers glucocorticoids secretion. It shows a basal release pattern that varies following a circadian cycle, with maximum values at sunrise and then descends until the minimum values at nightfall (Miller & Gronfier, 2006). In acute stress situations, this increase is produced quite more sharply, where the maximum increase takes place 20 to 40 minutes following the stressful situation (Dickerson & Kemeny, 2004).
With the purpose of conducting stress induction in a laboratory setting, several protocols have been designed, ranging from public speaking to arithmetical tests or stroop tasks (Moya-Albiol y Salvador, 2001). Along the same line, several studies have been carried out in order to demonstrate the implication of the serotonin transporter polymorphism in the cortisol response to stress, though contradictory results have been obtained (Bouma & Riese, 2010; Way & Taylor, 2010).
These differences could be explained by the influence of other factors that may affect cortisol release or sensitivity to stress, such as the nature of the stress inducing test that was used, the sample age or gender. It has been noticed that males evoke a more exaggerated response in stressful events (Uhart, Chong, Oswald, Lin & Wand, 2006). The results could also be affected by genotyping method used in the study, biallelic or triallelic, as no every study carries out genotyping of the sample by using the same method.
The large sample size that is needed, together with the high costs that this kind of studies involve, makes it difficult to conduct massive studies, therefore making meta-analysis become an interesting analysis tool, which can also prove possible causes of heterogeneity among studies (Munafo & Flint, 2004). With the purpose of providing information about the relation between the serotonin transporter polymorphism and sensitivity to stress mediated by cortisol, analysing at the same time how other influential factors affect this response, we have performed this meta-analysis.
Method
Search strategy and selection criteria
A systematic review of the literature published between 1980 and December 2011 was carried out through a search in the following databases: MEDLINE/PubMed, Embase, WOK and Psyinfo. The references cited by all relevant located studies were also examined. As search criteria, relevant terms were used, such as: "serotonin transporter polymorphism", "5-HTTLPR", "cortisol", "hydrocortisone", "stress". In the cases where relevant information was not available, authors were directly contacted via email. There were no restrictions in terms of language or publication status.
The selection criteria were defined as follows: studies where a stress-inducing intervention was undertaken and the study groups come defined by the genotype of the serotonin transporter. Studies undertaking different types of stress-inducing interventions were included.
The outcome must be the salivary cortisol concentration measured in different times during intervention. Studies which did not deviate from the Hardy-Weinberg equilibrium. Studies including patients with some kind of mental pathology or alteration of the hypothalamic-pituitary-adrenal axis at the time of the inclusion were excluded.
After the abstracts of all located references had been reviewed, the studies that did not meet the selection criteria were excluded and the texts of all potentially eligible articles were read entirety. Then, the articles were assessed separately, and relevant data were extracted and recorded in a data base created for this purpose. The dubious studies were resolved by the incorporation of an external expert.
Data extraction
According to the recommendations reported in meta-analytic methodology literature (Sánchez-Meca, Marin-Martinez and Lopez-Lopez, 2011) and in order to examine the influence of included studies on the effect size, the moderating variables of the included studies were coded considering the following aspects: patient characteristics, a) 5-HTTLPR genotype (s/s, s/l, l/l) b) Mean age (years), two studies reported the range of ages instead of the mean age, in these cases the mean of the range was calculated (Gotlib et al., 2010; Way & Taylor, 2010), c) % of males in the sample, d) race (Caucasian, African, Latin, mixed). Intervention characteristics: a) stress-inducing protocol (protocol name and emotional nature of protocol; negative, neutral, positive). Methodological characteristics: a) time of the experiment (morning, afternoon, both), b) sample size (number of subjects); c) SNP used (biallelic, triallelic).
As outcome variable of the selected studies, F and p genotype values and the sign of comparison (negative, positive) were gathered. In that cases where crude data were available, the mean area under the curve and the standard deviation were calculated.
Coding of moderator variables and outcome was conducted independently by two researchers. Additionally, an evaluation of the reliability of the coding process of moderating and outcome variables was carried out by calculating Cohen's kappa coefficient for qualitative variables, while for quantitative moderating variables was applied the intraclass correlation coefficient (ICC). Both coefficients reached a value of 1 for all variables.
Variables
The grouping variable was the 5-HTTLPR genotype. In order to carry out this meta-analysis, all data were grouped according to S' (s/s y s/l) and L' classification (l/l) in those studies that performed the analysis through a 5-HTTLPR s/l biallelic genotyping. In the studies in which triallelic geno-typing was carried out through 5-HTTLPR A/G polymorphism, they were subsequently grouped as follows: S' (La/Lg, La/S, Lg/Lg, Lg/S, S/S) and L' (La/La).
In the cases in which there were crude data, the mean and standard deviation of the areas under the curve obtained by trapezoidal formula (Pruessner, Kirschbaum, Meinlschmid & Hellhammer, 2003) were estimated for each study group. Before this, the possible outliers at each measurement moment were eliminated, which were defined as those values with a Z score ± 3. For those studies for which no crude data were obtained, the reported sign of the difference between groups and the F and p genotype values as magnitude measurement of the difference were taken. A negative sign was assigned in case of group L' was the one with the greatest area under the curve (higher response to stress) and a positive sign was assigned in case of group S' was the one with the greatest area under the curve.
In the studies where a possible interaction of the 5-HTTLPR polymorphisms with other factors in the response to stress was analyzed, the reported F or p values of such interaction were collected. Due to the lack of uniformity when carrying out the genotyping, the type of SNP used to genotyping was collected in order to analyze whether this had an impact on results. Moreover, the emotional nature of stress-inducing protocols, the mean age of the sample and the percentage of males in the included studies were collected.
Quality Assessment
To assess the quality of included studies, the Newcastle-Ottawa Scale for cohort studies was used (Wells et al., 2011). This scale assess the studies by the score obtained on eight questions that are distributed in three different sections corresponding to "selection", "comparability" and "outcome" with a score range of 1-9 (higher score - higher quality). An assessment of inter-observer reliability of the process of quality assessment was carried out by estimating the intra-class correlation coefficient (ICC) after comparing the scores obtained independently by two researchers. Mean ICC was .909 (range: .81-1).
Statistical analysis
For the statistical analysis, the software Comprehensive Metaanalysis Version 2.2.048 was used. In which the data referred to the F or p genotype values, the sign of difference and the sample size were included. Crude data were obtained from one study; the means and standard deviations were estimated and included in the analysis. All analyzes were carried out assuming a random effects model.
The effect of the type of polymorphism present was quantified by the standardised mean difference, (Hedges'g), as final estimator of the global effect size of all studies included.
Heterogeneity was analyzed through the x2 test and quantified by the I2 index (Higgins, Thompson, Deeks & Altman, 2003), with p values < .05 for the first and I2 > 50% for the last, as indicators of heterogeneity. In order to explain the heterogeneity by reference to continuous variables, a meta-regression was carried out based on the prior assumptions. This was the case for the variables "male percentage" and "average age" of the sample, which were incorporated to the analysis as explanatory variables and the effect size of each study estimated by the standardised mean difference, as a dependent variable (Egger, Smith & Altman, 2001). Whereas for the analysis of heterogeneity with reference to the qualitative variable "emotional nature of protocol", a subgroup analysis was carried out. Moreover, in order to determine whether the mean effects of the two subgroups of the variable "emotional nature of protocol" were statistically different from each other, an analysis through the statistical Qb was carried out.
The publication bias was studied by the application of Egger test and the construction of a Funnel Plot.
Results
As it can be observed in Figure 1, the search found a total of 758 references, of which 723 were directly excluded after analysing their respective abstracts or identify those duplicated, so there were 35 studies left for the reading of the complete text. The main reasons for the exclusion of the 723 studies revised were the following:
Initially, the studies were excluded after identifying, with the help of a reference manager, that they were duplicates and that they appeared in different databases consulted; others did not meet the requirement regarding the study design (revisions, letter to the editor, clinical cases, basic research); or were included in the study individuals who suffer from disorders in the Hypothalamic-Pituitary-Adrenal axis (alcoholism, depression, schizophrenia, several disorders); or were carried out in animals (monkeys, rats, fishes); in others, there was an intervention different from the one required (administration of drugs, Fmi studies) and in others, the outcome was not cortisol concentration or did not carry out the genotyping of the serotonin transporter polymorphism.
The texts of the remaining 35 studies were completely read. 24 out of these were excluded. The reasons for exclusion of these 24 studies were: 9 studies measured circadian cortisol; 1 study in which the cortisol response was measured during the performance of the heel prick in neonatal grouped according to their 5-HTTLPR polymorphism did not meet the Hardy-Weinberg Equilibrium (Mueller, Brocke, Fries, Lesch & Kirschbaum, 2010); 3 were duplicates, 1 was a review, in ó studies, intervention was not a psycho-social stress test. In 3 studies, patients with disorders in the Hypo-thalamic-Pituitary-Adrenal axis (alcoholism, and posttraumatic stress) were included, in 1 study; cortisol was not measured as an outcome.
Therefore, 11 studies met the preestablished selection criteria and were initially included in the systematic review (Alexander et al., 2009; Armbruster & Mueller, Moser, Lesch, Brocke & Kirschbaum, 2009; Bouma & Riese, 2010; Dougherty, Klein, Congdon, Canli & Hayden, 2010; Edelman et al., 2011; Frigerio et al., 2009; Gotlib, Joormann, Minor & Hallmayer, 2010; Mueller et al., 2011; Verschoor & Markus, 2011; Way & Taylor, 2010; Wust et al 2009) with 9 studies that were finally meta-analyzed (Alexander et al., 2009; Armbruster & Mueller, Moser, Lesch, Brocke & Kirschbaum, 2009; Bouma & Riese, 2010; Dougherty, Klein, Congdon, Canli & Hayden, 2010; Gotlib, Joormann, Minor & Hallmayer, 2010; Mueller et al., 2011; Verschoor & Markus, 2011; Way & Taylor, 2010; Wust et al 2009). Two studies were not finally meta-analyzed because, in one of them, the author did not provide us with the necessary data in order to include it in the meta-analysis and the other corresponding study did not report a F or p genotype value to be included in our meta-analysis.
The characteristics of the studies included in the analysis can be observed in Table 1.
In a study in which measurements were carried out both, in the morning and the afternoon, as there were crude data from this study, it was decided to analyze these data separately and obtaining a cohort for the morning and another cohort for the afternoon.
Study population
A total of 1715 individuals were analyzed in this metaanalysis. 1242 had S' genotype, while 473 had L' genotype.
In Table 2 detailed information about the sample analyzed in each study included in this meta-analysis is exposed.
In the study of Mueller et al. (2011), there were three sub-samples, as this study was made up of three cohorts of different age. In the study of Dougherty et al. (2010), there were two cohorts after separating crude data corresponding to measurements taken in the morning and afternoon.
In one of these studies (Gotlib et al. 2010), individuals whose mothers suffered or had suffered from any depression event were included.
The quality of studies included, estimated in a 1 to 9 scale had an average of 7.91 points (range: 7 to 9).
Quantitative analysis
Analysis by A/G SNP vs. analysis by S/L SNP
The reported results of those studies where the genotyping was carried out considering the biallelic method and the triallelic method were analyzed. No differences were obtained between both genotyping methods; SNP A/G (g - .112 (LI - - .229, LS - .454), p - .519) versus those studies where SNP S/L (g - .113 (LI - - .229, LS - .454), p - .518), analysing by random effects model. (Figure 2).
Main effect of the 5-HTTPLPR genotype
The Forest Plot showed, when analysing all cohorts included in the meta-analysis, that although there is a slight relation between the short polymorphism and higher cortisol concentrations, this is not statistically significant (g - .003 (LI - -.163, LS - .169), p - .975, assuming a random effects model). The analysis of heterogeneity showed that there was moderate heterogeneity (p - .015, I2 - 51.88%) among the studies analyzed. (Figure 3)
Subgroups analysis according to the emotional nature of the stress-inducing protocol
After detecting the heterogeneity previously mentioned, the cause was searched and in order to do this, the stress tests used in each study were carefully analyzed. A series of studies in which, cohorts were analyzed and exposed to a stress test within the context of psychosocial threat were located, whether through: a negative audience showing faces of boredom or dissatisfaction (Verschoor & Markus, 2011; Way & Taylor, 2010), or a procedure such as Lab-Tab (Dougherty et al., 2010) in which different emotions such as fear are induced, or the Edwart Social Competence (Gotlib et al. 2010), where individuals are asked to recall and describe an experience they consider to have been very stressful and, thus, very likely to have a high negatively emotional charge. This subgroup obtained significant results (g - .313 (LI - .101, LS - .525, p - .004) assuming a random effects model, with S' group showing higher Cortisol response than L' group. As regards heterogeneity found within this group, the values obtained (I2 - 0, p - .736) show lack of it.
On the other hand, we have analyzed those studies whose stress induction tests included the Trier Social Stress Task (Armbruster & Mueller et al., 2009; Mueller et al., 2011; Way & Taylor, 2010; Wüst et al., 2009), a speech to a neutral audience (Alexander et al., 2009) and the Groningen stress task (Bouma & Riese, 2010). This last study was finally included in this subgroup as, taking into consideration that the release of cortisol has a 20-minute delay after the stimulus; the sample corresponding to the second measurement taken in the study corresponds to the time of the speech, which is described as neutral. In sum, all these interventions were neutrally conducted without the induction of any negative emotion in the study participants. Within this subgroup, the results were not significant (g - -.149 (LI - -.335, LS - .037) p - .117). As regards the heterogeneity found within this group, values obtained (I2 - 46.083, p - .073) show a moderate heterogeneity. (Figure 4)
The analysis through the Qb statistics in order to assess the existence of significant differences among subgroups of the moderator variable "stress protocol emotional nature" (negative and neutral), showed a statistically significant result [Qb (1) - 10.294, p < .001; R2 - .295] and with an explained variance percentage of 29.5%. These results support those obtained above. Therefore, we can conclude more strongly the finding of a different cortisol response to stress in individuals belonging to S' group in comparison with those of L' group depending on the emotional context in which it appears. This different response to stress between both groups is significantly higher in a negative emotional context than in a neutral emotional context.
Interaction with other genes
After identifying studies that analyzed a possible interaction of the 5-HTTLPR polymorphism with other genes in the response to stress, two studies have been located. In one of them, there was an interaction with the gene of the D4 receptor (Armbruster & Mueller et al., 2009), this study published a significant interaction (F (1.84) -10.49, p - .002, if - .12). In the other study, there was a significant interaction with the gene BDNF (Slope: §; - .238, SE - .092, t (25) - 2.586, p - .016) and curve: §; = -.053, SE - .019, t (103) - -2.696, p - .009) being the homozygous for the short polymorphism and carriers of Met BDNF allele, the ones that showed the highest cortisol concentrations (Dougherty et al., 2010).
Interaction with previous stressful events
We have identified two studies that analyzed the effect of previous stressful events in interaction with the 5-HTTLPR polymorphism; both of them showed a significant interaction. In the study of Alexander et al. (2009), subjects homozygous for the short allele with a significant history of stressful life events exhibited markedly elevated cortisol secretions in response to the stressor compared to all other groups (F (1.93) - 8.3, p - .01, r f - .08) . In the study of Mueller et al. (2011), we have not observed this interaction in young adults (p > .69) and old people (p > .41), although it was observed in young adults (F (2.99) - 3.71; p - .028, r f - .07) for those stressful events suffered before they were five years old.
Interaction with age and gender
In this study, we have not found a significant interaction between the mean age of the sample and the 5-HTTLPR polymorphism in the cortisol response.
However, we have observed a negative trend with a 25% of variance explained by the model [bj - -.010; Qr(1) - 3.17, p - .075; R2 - .250] (Figure 5). Given this explained percentage of variance, the lack of statistical significance could be due to a problem of low statistical power because of the low number of studies.
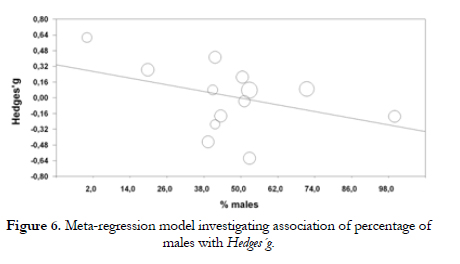
On the other hand, when studying the effect of the percentage of males in the sample, we have observed a slight negative trend although this was not significant in statistics terms with 22.7% of variance explained by the model (bj - -.006; Qr (1) - 2.15, p - .142; R2 - .227). (Figure 6).
Publication bias
The visual inspection of Funnel Plot when analysing standard errors against obtained g index, does not show evidence of publication bias. Egger test gives a p value > .05 so it does not show the presence of this bias either. (Figure 7).
Conclusions
Contrary to what was expected, the results of the global analysis did not support the hypothesis that the 5-HTTLPR short polymorphism is a sensitiveness factor for the cortisol response to stress. As this analysis had a significant heterogeneity, studies were examined in order to find a possible cause.
A possible cause of this heterogeneity was that the stress protocols used differed among the included studies; it was therefore necessary, to classify the included studies taking into consideration the nature of the stress protocols used. Simultaneously to a stress induction, some emotions such as fear or anxiety occurs and these emotions are caused by the situation assessment, which Lazarus (1993) identified as appraisal. For the assessment of the situation, individuals will use all the available clues, such as facial expressions of the audience participating in stress protocols, which has been observed to have an important impact on the participant's emotions (Sabatinelli et al. 2011). Lazarus identifies two types of appraisals; the primary appraisal is related to the assessment of the importance of an event and the secondary appraisal is more related to the interpretation of the result of such event in our welfare.
The importance given by participants of the studies protocols included is determined by egotype stimuli as indicated by authors of the Trier Social Stress Task (Kirschbaum, Pirke & Hellhammer, 1993) and it occurs similarly in the different studies. In the context of the secondary appraisal, that is, to give a more or less positive assessment of the event and its expectations, a negative perception of the situation has been induced in some studies, while in other studies a neutral intervention has been made which would result in different emotions.
These results show that individuals who carry the short allele 5-HTTLPR produce a significantly higher cortisol response to psycho-social stress when this stress has a negative emotional element. Likewise, in other study, we have observed how a higher score was obtained in the emotional assessment for negative experiences in those individuals carrying the short allele, after a description of a recent sad, scary and happy experience (Szily, Bowen, Unoka, Simon & Keri, 2008). In a meta-analysis of studies in which there was an induction of negative emotions, such as fear or a negative environment through negative facial expressions, it was observed a relation between the presence of the short allele and particularly, in individuals with homozygosis of this allele, with a higher reaction to emotions (Munafo et al., 2008). We have also observed a higher activation of the amygdala in homozygosis individuals for the short allele after showing a series of neutral and negative words to the sample (Canli T, Omura, Haas & Fallgatter, 2005).
In the subgroup defined by the studies where intervention was carried out in an emotionally neutral environment, individuals with short allele were those who showed the slightly smallest cortisol increase during the stressful task. These results show a significant difference in the implication of the 5-HTTLPR short allele depending on the environment where the stressful situation is taking place. This suggests a direct relation with the stress susceptibility hypothesis. There are two main hypotheses that explain this phenomenon. The diathesis-stress hypothesis (Monroe & Simons, 1991), according to which the individuals who have a certain vulnerability factor would be more susceptible to stress and to its effects than those individuals who do not have this factor when stress is experienced in a negative environment, while there would not be any difference between the individuals having and not having this factor in a more neutral or positive environment. The differential susceptibility hypothesis (Belsky, 2005), according to which there are some individuals who would present a negative or positive plasticity factor of susceptibility to stress depending on the environment being negative or positive.
In this context, studies about the interaction between short polymorphism of 5-HTTLPR gene and the environment as a possible susceptibility factor have been carried out. This conceptualisation of a negative or positive environment has been defined dissimilarly in different studies. While in some studies the negative environment was determined by vital stressful events (Caspi et al., 2003), in others it was defined by the socioeconomic status (Manuck, Flory, Ferrell, & Muldoon, 2004). It was also observed how the level of stress suffered along the day affected anxiety levels at the end of the day depending on the presence or not of the short allele or the Lg allele of gene 5-HTTLPR (Gunthert et al., 2007). The results of the subgroup of studies with no-negative interventions, did not show a significantly lower response by short allele carriers. In that case, it would have been in accordance with the theory that states the short allele as a plasticity genetic factor (Belsky & Pluess, 2009). This might be because those interventions were neutral; they did not take place in a positive environment. On the other hand, these results could be better explained by the diathesis-stress hypothesis whereby in a neutral environment, a similar response is expected for both, carriers and non-carriers of the vulnerability factor. However, providing answers to this question is not the purpose of this study. The results of this study support the hypothesis of a greater sensitivity to stress by the carriers of the short allele of 5-HTTLPR gene in negative stressful events; however, as we do not have data about situations in positive environments, we cannot completely relate our information with any hypothesis. In conclusion, this shows that short polymorphism is a factor involved in individual's susceptibility to stress, but instead of depending on the level of stress, it rather depends on the nature of stress.
Analysis of other possible moderating factors such as mean age or the percentage of male individuals in the sample did not bring significant statistical results. However, a strong trend was noticed when analysing the effect of the sample mean age. In samples with a lower mean age, S' group subjects showed a higher response to stress. And as the sample mean age went up, the L' group subjects were the ones who showed higher cortisol response to stress. Similarly, in the study of Mueller et al. 2011, differences were found regarding the effect of gene 5-HTTLPR polymorphism on cortisol response between the sample of children from 8 to 11 years old and the sample of adults from 19 to 31 years old. Higher responses were found also in L' group as the age went up. Nevertheless, due to the lack of statistical significance in these results, probably because of the small number of studies included in the analysis, this geneage interaction cannot be affirmed resolutely.
In relation with this phenomenon, it has been noticed how, as age increases, the availability of the serotonin transporter protein in the brain decreases (Van Dyck et al. 2000, Pirker et al. 2000). This indicates the existence of some agedependent factor that would be directly related with the expression level of 5-HTTLPR gene or with its result, with its subsequent effect in sensitivity to stress.
On the other hand, a slight trend was found towards a higher response to stress by individuals of the S' group in samples with a lower percentage of males. Meanwhile, in samples with a higher percentage of males, L' group subjects were the ones that showed a higher response to stress. These results are close to those found in previous studies (Wust et al., 2009, Jabbi et al., 2007). In addition, an interaction with previous stressful events has been noticed, probably via epigenetics. These environmental factors, if constant or excessive, could be the reason of a change process regarding the transcription capacity of some genes that could include genes of the serotoninergic system.
Given the complexity of the neuronal networks, it is logical to think about the participation of more than one type of neurotransmitters and brain zones in the elaboration of emotions and therefore in the activation of the HPA axis and subsequent cortisol release. In this review two studies have been found, which helped to confirm this hypothesis. It has been observed that the D4 dopamine receptor interacts in a significant manner with 5-HTTLPR and in the same way it has been observed that BDNF polymorphism interacts with 5-HTTLPR polymorphisms, reason for which it would be interesting to be able to repeat these studies and include these analysis in future studies.
This meta-analysis presents a series of limitations that must be taken into account. As it may be observed in the table about the characteristics of the studies analyzed, F genotype value in some studies was calculated by grouping the sample in s/s, s/l or l/l and in other studies S" or L\ In this respect, it has to be taken into account that the difference in the expected result must be low, as the group S' includes also s/s and s/l groups. To this, it should be added that every included study were in Hardy-Weinberg Equilibrium, as the frequency of the subjects of the three groups will be very similar in the different studies. Moreover, this meta-analysis includes studies on adults and none of them reported about the possible difference regarding the previous experience in talking in public of participants, which could distort the results. Therefore, these results must be interpreted cautiously.
In conclusion, the main result of this study confirms the role of the short polymorphism of 5-HTTLPR gen in the response to psychosocial stress, in the context of an emotionally negative environment. This polymorphism has been identified as an associated factor with a higher activity of the HPA axis resulting in a higher cortisol response. The results of this meta-analysis provide useful information about the etiology of the stress related pathologies, and suggest that this gene or its products could be included as possible therapeutic target for prevention or treatment. Future research comparing results in different contexts of social stress (negative, neutral and positive), considering possible confounding factors such as gender or age are recommended.
References
1. Alexander, N., Kuepper, Y., Schmitz, A., Osinsky, R., Kozyra, E., y Hennig, J. (2009). Gene-environment interactions predict cortisolresponses after acute stress: Implications for the etiology of depression. Psychoneuroendourinology, 34, 1294-1303. doi: 10.1016/j.psyneuen.2009.03.017. [ Links ]
2. Alexander, N., Osinsky, R., Schmitz, A., Mueller, E., Kuepper, Y., y Hennig, J. (2010). The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology, 35, 949-53. doi: 10.1016/j.psyneuen.2009.12.008. [ Links ]
3. Armbruster, D., y Mueller, A., Moser, D. A., Lesch, K. P., Brocke, B., y Kirschbaum, C. (2009). Interaction effect of D4 dopamine receptor gene and serotonin transporter promoter polymorphism on the cortisol stress response. Behavioral Neuroscience, 123, 1288-1295. [ Links ]
4. Ashcraft, K. A., Hunzeker, J., y Bonneau, R. H. (2008). Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology, 33, 951-63. doi: 10.1016/j.psyneuen.2008.04.010. [ Links ]
5. Belsky, J. (2005). Differential susceptibility to rearing influences: An evolutionary hypothesis and some evidence. In B. Ellis & D. Bjorklund (Eds.), Origins of the social mind: Evolutionary Psychology and Child Development (pp. 139-163). New York, NY: Guildford. [ Links ]
6. Belsky, J., y Pluess, M. (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin, 135, 885-908. [ Links ]
7. Bouma, E., y Riese, H. (2010). No replication of genotype effect of 5-HTTLPR on cortisol response to social stress in larger adolescent sample. Biological Psychiatry, 68, e33-e34. doi:10.1016/j.biopsych.2010.04.041. [ Links ]
8. Canli, T., Omura, K., Haas, B. W., Fallgatter, A., y Constable, R. T. (2005). Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of the National Academy of Sciences of the United States of America, 102, 12224-12229. doi: 10.1073/pnas.0503880102. [ Links ]
9. Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., McClay, J., Mill, J., Martin, J., Braithwaite, A., y Poulton, R. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science, 301, 386-389. doi: 10.1126/science.1083968. [ Links ]
10. Cohen, S., Tyrrell, D. A. J., y Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. The New England Journal of Medicine, 325, 606-61. [ Links ]
11. Dickerson, S. S., y Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355-391. doi: 10.1037/0033-2909.130.3.355. [ Links ]
12. Dougherty, L. R., Klein, D. N., Congdon, E., Canli, T., y Hayden, E. P. (2010) . Interaction between 5-HTTLPR and BDNF Val66Met polymorphisms on HPA axis reactivity in preschoolers. Biological Psychology, 83, 93. doi: 10.1016/j.biopsycho.2009.10.009. [ Links ]
13. Edelman, S., Shalev, I., Uzefovsky, F., Israel, S., Knafo, A., y Ebstein, R. P. (2011) . Epigenetic modification of the glucocorticoid receptor gene predicts women's salivary cortisol following a threat to the social self. European Neuropsychopharmacology, 21, S24-S25. doi: 10.1016/S0924-977X(11)70029-3. [ Links ]
14. Egger, M., Smith, G. D., y Altman, D. G. (2001). Systematic reviews in health care. Metaanalysis in context, 2nd ed, Cornwall: Bodmin. [ Links ]
15. Figueredo, V. M. (2009). The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. American Journal of Medicine, 122, 704-712. doi: 10.1016/j.amjmed.2009.05.001. [ Links ]
16. Frigerio, A., Ceppi, E., Rusconi, M., Giorda, R., Raggi, M. E., y Fearon, P. (2009). The role played by the interaction between genetic factors and attachment in the stress response in infancy. Journal of Child Psychology and Psychiatry, 50, 1513-1522. doi: 10.1111/j.1469-7610.2009.02126.x. [ Links ]
17. Gotlib, I. H., Joormann, J., Minor, K. L., y Hallmayer, J. (2010). HPA-Axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depresion. Psiquiatria Biológica, 17, 6-11. doi: 10.1016/j.biopsych.2007.10.008. [ Links ]
18. Gunthert, K., Conner, T., Armeli, S., Tennen, H., Covault, J., y Kranzler, H. (2007). Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: a daily process approach to geneenvironment interaction. Psychosomatic Medicine, 69, 762-768. doi: 10.1097/PSY.0b013e318157ad42. [ Links ]
19. Higgins, J. P., Thompson, S. G., Deeks, J. J., y Altman, D. G. (2003). Measuring inconsistency in meta-analyzes. British Medical Journal, 327, 557-60. doi: 10.1136/bmj.327.7414.557. [ Links ]
20. Ho, R. C. M., Neo, L. F., Chua, A. N. C., Cheak, A. A. C., y Mak, A. (2010). Research on psychoneuroimmunology: does stress influence immunity and cause coronary artery disease? Annals Academy of Medicine Singapore, 39, 191-196. [ Links ]
21. Hu, X. Z., Lipsky, R. H., Zhu, G., Akhtar, L. A., Taubmen, J., Greenberg B. D., y Goldman, D. (2006). Serotonin transporter gain offunction genotypes are linked to obsessive compulsive disorder. American Journal of Human Genetics, 78, 815-826. [ Links ]
22. Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, Ormel J, den Boer JA (2007). Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry, 12(5 ):483-90. [ Links ]
23. Jeckel, C. M. M., Lopes, R. P., Berleze, M. C., Luz, C., Feix, L., Argimon, I. I. D., Stein, L. M., y Bauer, M. E. (2010). Neuroendocrine and immunological correlates of chronic stress in 'strictly healthy' populations. Neuroimmunomodulation, 17, 9-18. doi: 10.1159/000243080. [ Links ]
24. Johnson, E. O., Kamilaris, T. C., Chrousos, G. P., y Gold, P. W. (1992). Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews, 16, 115-30. doi: 10.1016/S0149-7634(05)80175-7. [ Links ]
25. Karg, K., Burmeister, M., Schedden, K., y Sen, S. (2011). The serotonin transporter promoter variant (5-HTTLPR), stress and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry, 68, 444-454. doi:10.1001/archgenpsychiatry.2010.189. [ Links ]
26. Kirschbaum, C., Pirke, K. M., y Hellhammer, D. H. (1993). The 'Trier Social Stress Test'- a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76-81. [ Links ]
27. Kraft, J. B., Slager, S. L., McGrath, P. J., y Hamilton, S. P. (2005). Sequence analysis of the serotonin transporter and associations with antidepressant response. Biological Psychiatry, 58, 374-381. doi:10.1016/j.biopsych.2005.04.048. [ Links ]
28. Lazarus, R. S. (1993). Coping theory and research: Past, present, and future. Psychosomatic Medicine, 55, 234-247. [ Links ]
29. Manuck, S. B., Flory, J. D., Ferrell, R. E., y Muldoon, M. F. (2004). Socioeconomic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter genelinked polymorphic region. Psychoneuroendocrinology, 29, 651-668. doi: 10.1016/S0306-4530(03)00094-5. [ Links ]
30. Miller, M. W., y Gronfier, C. (2006). Diurnal variation of the startle reflex in relation to HPA-axis activity in humans. Psychophysiology, 43, 297-301. doi: 10.1111/j.1469-8986.2006.00400.x. [ Links ]
31. Moya-Albiol, L., y Salvador, A. (2001). Empleo de estrésores psicologicos de laboratorio en el estudio de la respuesta psicofisiologica al estrés. Anales de psicologia, 17, 69-81. [ Links ]
32. Monroe, S. M., y Harkness, K. L. (2005). Life stress, the "kindling" hypothesis and the recurrence of depression: considerations from a life stress perspective. Psychological Review, 112, 417-445. doi: 10.1037/0033-295X.112.2.417. [ Links ]
33. Monroe, S. M., y Simons, A. D. (1991). Diathesis-stress theories in the context of life-stress research: Implications for the depressive disorders. Psychological Bulletin, 110, 406-425. [ Links ]
34. Mueller, A., Armbruster, D., Moser, D. A., Canli, T., Lesch, K. P., Brocke, B., y Kirschbaum, C. (2011). Interaction of serotonin transporter gene-linked polymorphic region and stressful life events predicts cortisol stress response. Neuropsychopharmacology, 36, 1332-1339. doi:10.1038/npp.2011.11. [ Links ]
35. Mueller, A., Brocke, B., Fries, E., Lesch, K. P., y Kirschbaum, C. (2010). The role of the serotonin transporter polymorphism for the endocrine stress response in newborns. Psychoneuroendocrinology, 35, 289-296. doi: 10.1016/j.psyneuen.2009.07.002. [ Links ]
36. Munafo, M. R., Brown, S. M., y Hariri, A. R. (2008). Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry, 63, 852-857. doi:10.1016/j.biopsych.2007.08.016. [ Links ]
37. Munafo, M. R., y Flint, J. (2004). Meta-analysis of genetic association studies. Trends in Genetics, 20, 439-444. doi: 10.1016/j.tig.2004.06.014. [ Links ]
38. Pirker, W., Asenbaum, S., Hauk, M., Kandlhofer, S., Tauscher, J., Willeit, M., Neumeister, A., Praschak-Rieder, N., Angelberger, P., Brucke, T (2000). Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. Journal of Nuclear Medicine, 41 (1):36-44. [ Links ]
39. Post, R. M. (1992). Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry, 149, 999-1010. [ Links ]
40. Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., y Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916-931. doi: 10.1016/S0306-4530(02)00108-7. [ Links ]
41. Sabatinelli, D., Fortune, E. E., Qingyang, L., Siddiquib, A., Krafftb, C., William, T., Beck, S., y Jeffries, J. (2011). Emotional perception: Meta-analyzes of face and natural scene processing. Neuroimage, 54, 2524-2533. doi: 10.1016/j.neuroimage.2010.10.011. [ Links ]
42. Sánchez-Meca, J., Marin-Martínez F., y López-López J. A. (2011). Meta-análisis e intervención Psicosocial Basada en la Evidencia. Psychosocial Intervention, 20, 95-107. doi: 10.5093/in2011v20n1a9. [ Links ]
43. Shalev, I., Lerer, E., Israel, S., Uzefovsky, F., Gritsenko, I., Mankuta, D., Ebstein, R. P., y Kaitz, M. (2009). BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology, 34, 382-8. doi: 10.1016/j.psyneuen.2008.09.017. [ Links ]
44. Silva, H., Iturra, P., Solari, A., Villarroel, J., Jerez, S., Roa, N., y Bustamante, M. L. (2010). Respuesta a fluoxetina y polimorfismos del transportador de la serotonina en trastorno límite de personalidad. Revista Chilena de Neuro-Psiquiatría, 48, 29-37. doi: 10.4067/S0717-92272010000200004. [ Links ]
45. Steptoe, A., Van Jaarsveld, C. H., Semmler, C., Plomin, R., y Wardle, J. (2009). Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology, 34, 273-80. doi: 10.1016/j.psyneuen.2008.09.006. [ Links ]
46. Szily, E., Bowen, J., Unoka, Z., Simon, L., y Keri, S. (2008). Emotion appraisal is modulated by the genetic polymorphism of the serotonin transporter. Journal of Neural Transmission, 115, 819-22. doi: 10.1007/s00702-008-0029-4. [ Links ]
47. Uhart, M., Chong, R. Y., Oswald, L., Lin, P. I., y Wand, G. S. (2006). Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology, 31, 642-52. doi: 10.1016/j.psyneuen.2006.02.003. [ Links ]
48. Van Dyck C.H., Malison R.T., Seibyl J.P., Laruelle M., Klumpp H., Zoghbi S.S., Baldwin R.M., Innis R.B (2000). Agerelated decline in central serotonin transporter availability with ((123)I) beta-CIT SPECT. Neurobiology of Aging, 21(4), 497-501. doi: 10.1016/S0197-4580(00)00152-4. [ Links ]
49. Verschoor, E., y Markus, C. R. (2011). Effects of acute psychosocial stress exposure on endocrine and affective reactivity in college students differing in the 5-HTTLPR genotype and trait neuroticism. Stress, 14, 407-419. [ Links ]
50. Way, B. M., y Taylor, S. E. (2010). The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry, 67, 487-492. doi:10.1016/j.biopsych.2009.10.021. [ Links ]
51. Way, B. M., y Taylor, S. E. (2010). Reply to: No replication of genotype effect of 5-HTTLPR on cortisol response to social stress in larger adolescent sample. Biological Psychiatry, 68, e35. [ Links ]
52. Wells, G., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., y Tugwell, P (2011). Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta analyses. Recuperada de http://www.ohri.ca/programs/clinicalepidemiology/ oxford.asp. [ Links ]
53. Wust, S., Kumsta, R., Treutlein, J., Frank, J., Entringer, S., Schulze, T. G., y Rietschel, M. (2009). Sex-specific association between the 5-HTT genelinked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology, 34, 972-982. doi: 10.1016/j.psyneuen.2009.01.011. [ Links ]
![]() Correspondence:
Correspondence:
Emiliano Agüero Tejado.
Institution: Clinical Research Unit,
University Hospital of Torrejón de Ardoz.
Correspondence: C/Mateo Inuma, s/n.
28850 Torrejón de Ardoz, Madrid (Spain).
E-mail: eaguero@torrejonsalud.com
Article received: 17-5-2012
Reviewed: 4-5-2013
Accepted: 19-12-2013