Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Anales de Psicología
versión On-line ISSN 1695-2294versión impresa ISSN 0212-9728
Anal. Psicol. vol.32 no.1 Murcia ene. 2016
https://dx.doi.org/10.6018/analesps.32.1.182331
Cognitive Reserve Scale and ageing
Escala de Reserva Cognitiva y envejecimiento
Irene León, Juan García-García and Lola Roldán-Tapia
Department of Psychology, University of Almeria, Almeria (Spain).
This work was supported by the Ministry of Economy and Competitiveness [PSI2011-26985] and a Plan Propio de investigación grant to IL (University of Almeria).
ABSTRACT
The construct of cognitive reserve attempts to explain why some individuals with brain impairment, and some people during normal ageing, can solve cognitive tasks better than expected. This study aimed to estimate cognitive reserve in a healthy sample of people aged 65 years and over, with special attention to its influence on cognitive performance. For this purpose, it used the Cognitive Reserve Scale (CRS) and a neuropsychological battery that included tests of attention and memory. The results revealed that women obtained higher total CRS raw scores than men. Moreover, the CRS predicted the learning curve, short-term and long-term memory, but not attentional and working memory performance. Thus, the CRS offers a new proxy of cognitive reserve based on cognitively stimulating activities performed by healthy elderly people. Following an active lifestyle throughout life was associated with better intellectual performance and positive effects on relevant aspects of quality of life.
Key words: Activities; quality of life; lifestyle; elderly; measurement; neuropsychology; cognitive reserve.
RESUMEN
El constructo de reserva cognitiva intenta explicar por qué algunos sujetos con patología cerebral o durante el proceso de envejecimiento normal pueden ejecutar tareas cognitivas a un nivel superior al esperado. Los objetivos del presente estudio abarcan la estimación de reserva cognitiva en sujetos sanos a partir de los 65 años y su repercusión a nivel cognitivo. Para ello, se empleó la Escala de Reserva Cognitiva (ERC) y una batería neuropsicológica que englobaba tareas mnésicas y atencionales. Los resultados revelaron que las mujeres obtuvieron mayor puntuación directa en la ERC. Además, la ERC predijo la ejecución en tareas de memoria (curva de aprendizaje y recuerdos a corto plazo y a largo plazo), pero no predijo las puntuaciones en memoria de trabajo ni en atención. Así, la ERC ofreció una estimación de reserva cognitiva, basada en el estilo de vida, en sujetos sanos mayores. Mantener un estilo de vida activo a lo largo de los años favorece la ejecución intelectual y repercute positivamente en facetas relevantes para la calidad de vida.
Palabras clave: Actividades; calidad de vida; estilo de vida; mayores; medición, neuropsicología; reserva cognitiva.
Introduction
In Spain, according to the National Institute of Statistics (Instituto Nacional de Estadistica, 2013), life expectancy for individuals older than 65 years increased during the period 1992-2011, by 2.5 years for men and 2.7 years for women. In addition, increases of .8 years and 1.1 years, respectively, were seen for men and women aged 85 years upwards.
Associated with this increased life expectancy, many efforts are being made to maintain people's quality of life. In this sense, according to the World Health Organization (2013), the lack of disease is complemented by the promotion of physical, social and emotional factors. It has been demonstrated that following an active lifestyle, which means incorporating cognitively stimulating, physical and social activities, contributes not only to delayed cognitive impairment related to ageing but also to delaying the onset of dementia (Fratiglioni, Paillard-Borg, & Winblad, 2004; Paillard-Borg, Fratiglioni, Xu, Winbland, & Wang, 2012; Sobral & Paul, 2013; Wilson et al., 2013).
In this regard, a range of studies has focused on the quantification of these types of activities and on analysing their influence on brain function and structure and neuropsychological performance (Sánchez, Torrellas, Martín, & Barrera, 2011; Valenzuela & Sachdev, 2007; Valenzuela, Sachdev, Wen, Chen, & Brodaty, 2008). Vemuri et al. (2012) studied 515 nondemented subjects and found that the intellectual activity (lifetime and current) and the biomarkers Alzheimer's disease pathophysiology (amyloid burden, glucose metabolism and hippocampal volume) may explain the observed variability in cognitive performance. No associations were found between these biomarkers and physical and intellectual activities.
The construct of cognitive reserve may explain the benefits, especially the cognitive ones, of spending time on brain stimulation in leisure, social and cognitive activities. Cognitive reserve could explain the findings that clinical manifestations of brain damage were delayed, and that subjects without brain lesions and with high cognitive reserve perform more successfully than those with low cognitive reserve (Stern, 2009). Thus, a wide variety of cognitive reserve studies has focused on healthy ageing and dementia (Ewers, Insel, Stern, & Weiner, 2013; Premi et al., 2013; Stern, 2012).
The concept of cognitive reserve is understood differently in relation to the model followed: cognitive reserve or cerebral reserve. According to the cognitive reserve model, inter-individual differences in cognitive processes may explain better performance in both healthy individuals and patients with neuropathology. Regarding the cerebral reserve model, inter-individual differences can be registered in the capacity to accumulate brain pathology before reaching a certain threshold above which clinical manifestations appear. The present study follows the cognitive reserve model.
The registration of stimulating cognitive activities throughout a person's lifetime is one way to measure cognitive reserve (León, García, & Roldán-Tapia, 2011; Nucci, Mapelli, & Mondini, 2012; Rami et al., 2011; Valenzuela & Sachdev, 2007; Wilson, Barnes, & Bennett, 2003). In this perspective, a new test called the Cognitive Reserve Scale (CRS) has been developed to estimate cognitive reserve in the Spanish population (Leon, García-García, & Roldán-Tapia, 2014). The CRS contains 24 ítems and is divided into three periods of life: young adulthood, adulthood and late adulthood. Consequently, it is possible to obtain a CRS total score and three partial scores corresponding to each period of life.
It has been demonstrated that cognitive reserve is flexible and it may change during the lifespan (Rodríguez-Álvarez & Sánchez-Rodríguez, 2004; Tucker & Stern, 2011). Therefore, all the efforts made individually and by institutions to potentiate education and stimulating activities, and to promote environmental enrichment, may have positive effects on the individual's quality of life and for society in general, including economic benefits.
The goals of the present study were: (1) to estimate cognitive reserve using the CRS in healthy individuals older than 65 years; and (2) to study the relation between CRS scores and cognitive performance.
Method
Subjects
Thirty healthy subjects participated in this study (22 females, 8 males). The age ranged between 65 and 88 years (Mean = 72.9, SD = 6.04). Table 1 shows the characteristics of the participants. The education variable was measured as years of education (quantitative), and as low (< 8 years) or high (> 8 years) educational attainment (semi-quantitative). Occupational attainment was classified in two steps: first, the professions were categorized according to the National Classification of Occupations of the National Institute of Statistics (Instituto Nacional de Estadística, 2011); and secondly, they were split into high, medium or low occupational attainment (1, 2, 3) in relation to the qualification required. Thus, 1 was for managers, scientific and intellectual technicians and professionals; 2 for clerks, accountants and related professionals, and professionals in the armed forces; and 3 for sales agents and customer service employees, skilled workers in the agriculture, forestry and fishery industry, workers in crafts and related trades, plant and machine operators, manual occupations and home-makers.The exclusion criteria included a clinical history of psychiatric or neurological disorders, drug consumption, a score of < 27 on the Mini Examen Cognoscitivo (MEC) (Lobo, Esquerra, Gómez-Burgada, Sala, & Seva, 1979), a Spanish versión of the MiniMental State (Folstein, Folstein, & McHugh, 1975), and uncorrectable hearing and/or visual impairment.
All subjects were volunteers and they signed written consent forms. This study was conducted in compliance with the Declaration of Helsinki and Spanish legislation on personal data protection.
Instruments
Cognitive Reserve Scale
The CRS is a proxy of cognitive reserve focused on the Spanish population. The psychometric properties of the CRS suggest its adequacy to measure cognitive reserve (Leon et al., 2014).
The CRS registers the frequency of cognitively stimulating activities carried out across the lifetime. A total of 24 ítems are distributed into four aspects: activities of daily living, training/information, hobbies and social life. In addition, the CRS is divided into three periods of life: young adulthood (18-35 years), adulthood (36-64 years) and late adulthood (> 65 years).
To measure the frequency of the activities included in the CRS, a five-point Likert scale was designed, where 0 = never and 4 = three or more times a week, whenever I have the opportunity. This procedure facilitated the application of the scale and the calculation of the scores. Thus, each subject answered each item several times, according to the established age periods (young adulthood, adulthood and late adulthood). Total CRS score and partial scores for each age stage were the result of summing raw scores. The median of the total CRS score was used to classify participants into high and low cognitive reserve (Scarmeas, Levy, Tang, Manly, & Stern, 2001).
The study of the psychometric properties of the CRS revealed an adequate reliability (Cronbach's alpha = .77). Validity evidence was obtained through different methods. First, a review of cognitive reserve studies was conducted to generate the ítems of the scale, and the CRS was supervised by experts (León et al., 2011). Secondly, the relationships between the CRS score and educational attainment, occupational attainment and IQ, three variables usually used as proxies of cognitive reserve (León et al., 2014), were analysed. The results showed that educational attainment affected the CRS score (p = .004, partial η2 = .07), and that neither occupational attainment (p = .898) nor IQ (p = .33) affected the CRS score. Finally, validity evidence was also found by analysing the theoretical relationships between CRS score and cognitive performance. The total CRS score correlated with memory, abstract reasoning, and visuoconstruction and visuoperception tasks.
Neuropsychological assessment
The Vocabulary subtest (Wechsler, 2004) was administered to obtain a measure of verbal premorbid intelligence (IQ), and the MEC score (Lobo et al., 1979) was used as an exclusion criterion if it was less than 27. The rest of the neuropsychological tasks were categorized into three cognitive domains: memory, working memory and attention. The memory tasks were divided into verbal material: TAVEC (trials 1-5, short-term and long-term memory, and recognition) (Benedet & Alejandre, 1998); and non-verbal material: Rey-Osterrieth Complex Figure (ROCF; short-term and long-term memory) (Rey, 2009); the Digit Span subtest (backward) (Wechsler, 2004) and Corsi's Block-Tapping test (backward) (Tamayo et al., 2012) were used to estimate working memory. In relation to attention, the Digit Span subtest (forward) (Wechsler, 2004), Corsi's Block-Tapping (forward) and the Stroop test (Golden, 2010) were administered.
Procedure
Several associations and social centres in Almeria were visited to provide information about the objectives of the study and to recruit volunteers. The assessment session was conducted individually by a trained psychologist and took approximately 1.5 hours. Sociodemographic characteristics and medical information were registered in the initial interview; then, the MEC (Lobo et al., 1979) and Vocabulary subtest (Wechsler, 2004) were administered; and finally, the rest of the neuropsychological tasks and the CRS. All participants were provided with information about the session, and confidentiality and anonymity of the collected data were guaranteed.
Data analysis
Analyses were carried out with the statistical package SPSS (versión 21.0). The education variable was treated as both continuous (years of education) and dichotomous (low and high). The median of the CRS total raw scores (154.5) was the criterion used to divide subjects into low (< 154.5) and high (> 154.5) cognitive reserve.
The Student's t test was used to study gender differences among quantitative variables, while the chi-squared (χ2) test and Fisher's exact test were used to compare quasiquantitative variables (educational and occupational attainments). Pearson's correlation was used to analyse the relationship between IQ and the CRS total score.
A two-way ANOVA (gender x time) with repeated measures in the last factor was applied. The three levels of the variable time' corresponded to young adulthood, adulthood and late adulthood. In this analysis, the assumption of sphericity was analysed, the variable education (years) was controlled and post-hoc analyses were conducted.
In addition, MANOVA was used to analyse the influence of CRS score (low/high) on cognitive performance, controlling for education (years).
The size effect was estimated using partial eta-squared (partial η2) and was interpreted as low = .01, medium = .059 or large = .138 (Cohen, 1988).
Results
The mean age of the sample was 72.9 years (SD = 6.04) and the educational attainment ranged from no education to 21 years (Mean = 9.63, SD = 5.021). Table 1 shows the sociodemographic characteristics of the participants. All individuals were independent in activities of daily living (Barthel Index). In relation to educational attainment, the distribution of females was similar between low and high (54.5% and 45.5%, respectively), while a higher percentage of males than females had completed more than 8 years of education (75%). Regarding occupational attainment, the distribution of males was similar for high, medium and low (25%, 37.5% and 37.5%, respectively). A larger percentage of females than males had less intellectually demanding professions (63.6%).
The analysis revealed no statistically significant differences between genders in age [t(28) = -.62, p = .539], educational attainment (years) [t(28) = 1.15, p = .259], educational attainment (low/high) [χ2 = 2.06, p = .226], occupational attainment [χ2 = 2.4, p = .323], verbal IQ [t(28) = 1.02, p = .318] or Barthel Index [t(28) = .6, p = .556]. However, males showed higher MEC scores than females [t(28) = 2.44, p = .022, rbp = .315]. The effect size was moderate (Table 1).
Cognitive Reserve Scale
No correlation was registered between verbal IQ and CRS total score (r = .14, p = .455) or between verbal IQ and the partial CRS scores: young adulthood (r = .07, p = .72), adulthood (r = .24, p = .207) and late adulthood (r = .08, p = .683).
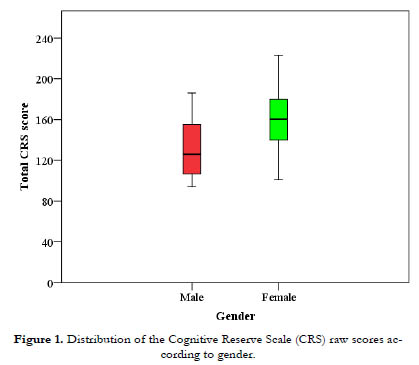
For the repeated-measures variable "time", the assumption of sphericity was not met (p = .048), so Greenhouse-Geisser correction was applied. Analysis revealed no interaction between time and gender [F(1.66, 44.7) = .663, p = .493]; however, significant main effects were observed for time and gender, with large size effects (partial η2 p > .138). In relation to gender [F(1,27) = 9.72, p = .004, partial η2 = .265], females (mean = 159.64, SD = 27.85) showed higher scores than males (mean = 131.88, SD = 31.63) (Figure 1). Regarding time [F(1.66, 44.7) = 6.07, p = .007, partial η2 = .183], post-hoc analysis and Bonferroni correction revealed significant differences among young adulthood (mean = 41.72, SD = 2.41), adulthood (mean = 48.04, SD = 2.01) and late adulthood (mean = 54.5, SD = 1.83) (Figure 2).
Cognitive performance and the Cognitive Reserve Scale
The CRS score predicted memory performance assessed using verbal material. The first TAVEC trial did not achieve statistical significance [F(1,27) = 2.74, p = .11], whereas statistical significance was observed for trials 2-5 [trial 2, F(1,27) = 21.56, p < .000, partial η2 = .44; trial 3, F(1,27) = 10.43, p = .003, partial η2 = .28; trial 4, F(1,27) = 6.04, p = .021, partial η2 = .18; and trial 5, F(1,27) = 8.21, p = .008, partial η2 = .233], short-term recall [F(1,27) = 8.14, p = .008, partial η2 = .23], long-term recall [F(1,27) = 13.98, p = .001, partial η2 = .34] and recognition [F(1,27) = 6.13, p = .02, partial η2 = .19]. The effect sizes were large (partial η2 > .138).
Analysis of memory measured by non-verbal material showed that the CRS score predicted short-term [F(1,26) = 12.23, p = .002, partial η2 = .32] and long-term memory [F(1,26) = 11.19, p = .003, partial η2 = .3]. Large effect sizes were observed (partial η2 > .138).
The CRS did not predict attentional performance [F(5,23) = 1.33, p = .287] or working memory [F(2,26) = .78, p = .47].
Discussion
The results showed that females spent more time than males performing cognitively stimulating activities. The CRS score predicted the cognitive performance related to memory tasks.
In the past few decades, the political and social context has changed notably in Spain, as well as the roles played by males and females. The age of the participants in the study ranged between 65 and 88 years; therefore, they were born in the 1920s, 1930s and 1940s. During these decades and well into the twenty-first century, males spent most of their time in professional or other occupations, whereas females were mainly dedicated to bringing up children and doing housework. This factor may have an effect on the CRS score and could explain why the women took part in more social, intellectual and leisure activities.
In this study, all subjects were healthy and had MEC (Lobo et al., 1979) scores < 27, with males outperforming females. Moreover, Barthel Index scores revealed that all the individuals were independent in their activities of daily living.
The analysis revealed statistically significant differences in relation to years of education, which ranged from none to 21 years. Recently, it has been demonstrated that those individuals with more educational years show increased cerebral connectivity and more regional volume of the cerebral cortex (Arenaza-Urquijo et al., 2013; Liu et al., 2012). Among Alzheimer's disease patients, more education has been associated with a faster decline in certain cognitive domains, such as abstract reasoning, but at the same time it may help to conserve other cognitive performance (Le Carret et al., 2005). A low educational attainment may increase the risk of developing dementia (Caamaño-Isorna, Corral, Montes-Martínez, & Takkouche, 2006; see meta-analysis by Meng & D'Arcy, 2012), whereas a high educational attainment may help to delay the clinical manifestation of brain damage (Carnero-Pardo & del Ser, 2007; Staff, Murray, Deary, & Whalley, 2004). However, not all studies have found education to be a protective factor against the cognitive decline related to ageing (Van Dijk, Van Gerven, Van Boxtel, Van der Elst, & Jolles, 2008).
In the present study, taking into consideration the political and social context of the participants, women did less cognitively demanding occupational activities throughout their lifetime (63.6%), including housework, than men. Suo et al. (2012) demonstrated that hippocampal atrophy is smaller in those subjects who had supervisory and directive roles in their occupational activity. Besides, individuals with high occupational attainment have a reduced risk of developing dementia and this factor contributes to the maintenance of cognitive execution during ageing (Staff et al, 2004; Stern, 2012).
On the other hand, male and female participants showed similar verbal IQ distributions. No relation was found between verbal IQ and total and partial CRS scores. It should be noted that the IQ was assessed by a cognitive task, whereas the cognitive reserve is a different construct measured using a non-cognitive task (Nucci et al., 2012) in the present study.
High and low cognitive reserve estimated using the median of the CRS raw scores predicted memory performance with verbal and non-verbal material, as well as the learning curve, in healthy individuals older than 65 years. However, the data showed that cognitive reserve may not affect significantly attention and working memory. Previously, Roldán-Tapia, García, Canovas, and Leon (2012) found that individuals with high reserve outperformed cognitively those with low reserve, including on attentional and working memory tasks. Nevertheless, the proxy of cognitive reserve used was based on a sum of IQ, educational and occupational attainment, not considering the perspective of the frequency of stimulating activities.
In the present study, cognitive reserve was estimated using the CRS, a test that reflects the frequency of participation in different cognitive, social and leisure activities across the lifetime. Women spent more time than men in these brain-stimulating activities. The delay of the onset of symptomatology associated with dementia is related to a better quality of life in elderly people, and it has been demonstrated that performing stimulating cognitive activities across the lifespan contributes to this (Akbaraly et al., 2009). A more active lifestyle is also related to increased cognitive plasticity (Calero, Navarro, & Muñoz, 2007), more functional independency and more autonomy in activities of daily living among elderly people (Navarro, Calero, López, Gómez, Torres, & Calero, 2008).
However, does the participation in stimulating activities explain cognitive performance, or could it be that those with better cognition participate more frequently in intellectual, physical and social activities? (Díaz-Orueta, Buiza-Bueno, & Yanguas-Lezaun, 2010; Scarmeas, 2007). This directional duality has been investigated by the longitudinal Victoria study (Small, Dixon, McArdle, & Grimm, 2012). After a follow-up period of 12 years, the conclusion reached was not only that lifestyle provides protection from certain cognitive changes in ageing, but also that impaired cognitive execution reduces engagement with stimulating activities.
In general, the maintenance of an active lifestyle leads to successful ageing (Hertzog, Kramer, Wilson, & Linden-berger, 2008), but the normal ageing process is also affected by a wide variety of factors such as genetics, general health, psychological well-being and attitude to old age, as well as environmental stimulation, socioeconomic resources and social politics.
The study has some limitations. The selection of the sample was not random; therefore, in future investigations it would be advisable to choose a technique that ensures the representativeness of the sample. Additionally, the sample size was small and fewer men than women agreed to participate.
There has been a remarkable level of interest in research on cognitive reserve from diverse scientific disciplines. Political and social concerns are also reflected in the implementation of interventional strategies to contribute to the delay of neuropathological symptoms and achieve a better quality of life.
Acknowledgements
The authors thank the participants in the study.
References
1. Akbaraly, T. N., Portet, F., Fustinoni, S., Dartigues, J. F., Artero, S., Rouaud, O., ... Berr, C. (2009). Leisure activities and the risk of dementia in the elderly: Results from the three-city study. Neurology, 73, 854-861. [ Links ]
2. Arenaza-Urquijo, E. M., Landeau, B., La Joie, R., Mevel, K., Mézenge, F., Perrotin, A., ... Chételat, G. (2013). Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage, 83, 450-457. [ Links ]
3. Benedet, M., & Alejandre, M. (1998). Test de aprendizaje verbal España-Complutense (TAVEC). Madrid: TEA. [ Links ]
4. Caamaño-Isorna, F., Corral, M., Montes-Martínez, A., & Takkouche, B. (2006). Education and dementia: A meta-analytic study. Neuroepidemiology, 26, 226-232. [ Links ]
5. Calero, M. D., Navarro, E., & Muñoz, L. (2007). Influence of activity on cognitive performance and cognitive plasticity in elderly persons. Archives of Gerontology and Geriatrics, 45, 307-318. [ Links ]
6. Carnero-Pardo, C., & del Ser, T. (2007). La educación proporciona reserva cognitiva en el deterioro cognitivo y la demencia. Neurología, 22, 78-85. [ Links ]
7. Cohen, J. (1988). Statistical power analysis for the behavioural sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [ Links ]
8. Díaz-Orueta, U., Buiza-Bueno, C., & Yanguas-Lezaun, J. (2010). Reserva cognitiva: Evidencias, limitaciones y líneas de investigación futura. Revista Española de Geriatría y Gerontología, 45, 150-155. [ Links ]
9. Ewers, M., Insel, P. S., Stern, Y., & Weiner, M. W. (2013). Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology, 80, 1194-1201. [ Links ]
10. Folstein, M., Folstein, S. E., & McHugh, P. R. (1975). Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189-198. [ Links ]
11. Fratiglioni, L., Paillard-Borg, S., & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. Neurology, 3, 343-353. [ Links ]
12. Golden, C. J. (2010). Stroop: Test de colores y palabras. Madrid: TEA. [ Links ]
13. Hertzog, C., Kramer, A. F., Wilson, R. S., & Lindenberger, U. (2008). Enrichment effects on the adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, Supplement, 9, 1-65. [ Links ]
14. Instituto Nacional de Estadística (2011). Clasificación Nacional de Ocupaciones (CNO). Retrieved from http://www.ine.es/jaxi/menu.do?type=pcaxis&path=/t40/cno11&file=inebase. [ Links ]
15. Instituto Nacional de Estadística (2013). España en cifras. Retrieved from http://www.ine.es/ss/Satellite?L=es_ES&c=INEPublicacion_C&cid=1259924856416&p=1254735110672&pagename=ProductosYServicios%2FPYSLayout¶m1=PYSDetalleGratuitas. [ Links ]
16. Le Carret, N., Auriacombe, S., Letenneur, L., Bergua, V., Dartigues, J. F., & Fabrigoule, C. (2005). Influence of education on the pattern of cognitive deterioration in AD patients: The cognitive reserve hypothesis. Brain and Cognition, 57, 120-126. [ Links ]
17. León, I., García, J., & Roldán-Tapia, L. (2011). Construcción de la Escala de Reserva Cognitiva en población española: Estudio piloto (Development of the Scale of Cognitive Reserve in Spanish population: A pilot study). Revista de Neurología, 52, 653-660. [ Links ]
18. León, I., García-García, J., & Roldán-Tapia, L. (2014). Estimating cognitive reserve in healthy adults using the Cognitive Reserve Scale. PEoS One, 9: e102632. doi:10.1371/journal.pone.0102632. [ Links ]
19. Liu, Y., Julkunen, V., Paajanen, T., Westman, E., Wahlund, L. O., Aitken, A., ... Soininen, H. (2012). Education increases reserve against Alzheimer's disease: Evidence from structural MRI analysis. Neuroradiology, 54, 929-938. [ Links ]
20. Lobo, A., Esquerra, J., Gómez-Burgada, F., Sala, J. M., & Seva, A. (1979). El Mini-Examen Cognoscitivo: Un test sencillo y práctico para detectar alteraciones intelectuales en pacientes médicos. Actas Luso-españolas de Neurología, Psiquiatría y Ciencias afines, 3, 189-202. [ Links ]
21. Meng, X., & D'Arcy, C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with metaanalyses and qualitative analyses. PEoS One, 7: e38268. doi: 10.1371/journal.pone.0038268. [ Links ]
22. Navarro, E., Calero, M. D., Lopez, A., Gómez, A. L., Torres, I., & Calero, M. J. (2008). Nivel de independence en la vida diaria y plasticidad cognitiva en la vejez. Escritos de Psicología, 2, 74-84. [ Links ]
23. Nucci, M., Mapelli, D., & Mondini, S. (2012). Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clinical and Experimental Research, 24, 218-226. [ Links ]
24. Paillard-Borg, S., Fratiglioni, L., Xu, W., Winbland, B., & Wang, H. X. (2012). An active lifestyle postpones dementia onset by more than one year in very old adults. Journal of Alzheimer's Disease, 31, 835-842. [ Links ]
25. Premi, E., Garibotto, Y., Gazzina, S., Grassi, M., Cosseddu, M., Paghera, B., ... Borroni, B. (2013). Beyond cognitive reserve: Behavioural reserve hypothesis in frontotemporal dementia. Behavioural Brain Research, 15, 245-262. [ Links ]
26. Ramí, L., Valls-Pedret, C., Bartrés-Faz, D., Caprile, C., Solé-Padullés, C., Castellví, ... Molinuevo, J. L. (2011). Cuestionario de reserva cognitiva. Valores obtenidos en población anciana sana y con enfermedad de Alzheimer (Cognitive reserve questionnaire. Scores obtained in a healthy elderly population and in one with Alzheimers disease). Revista de Neurología, 52, 195-201. [ Links ]
27. Rey, A. (2009). Test de copia de una figura compleja. Madrid: TEA. [ Links ]
28. Rodríguez-Álvarez, M., & Sánchez-Rodríguez, J. L. (2004). Reserva cognitiva y demencia. Anales de Psicología, 20, 175-186. [ Links ]
29. Roldán-Tapia, L., García, J., Canovas, R., & León, I. (2012). Cognitive reserve, age, and their relation to attentional and executive functions. Applied Neuropsychology: Adult, 19, 2-8. [ Links ]
30. Sánchez, J. L., Torrellas, C., Martín, J., & Barrera, I. (2011). Study of sociodemographic variables linked to lifestyle and their possible influence on cognitive reserve. Journal of Clinical and Experimental Neuropsychology, 33, 874-891. [ Links ]
31. Scarmeas, N. (2007). Lifestyle patterns and cognitive reserve. In Y. Stern (Ed.), Cognitive reserve (pp. 187-206). New York: Taylor & Francis. [ Links ]
32. Scarmeas, N., Levy, G., Tang, M. X., Manly, J., & Stern Y. (2001). Influence of leisure activity on the incidence of Alzheimer's disease. Neurology, 57, 2236-2242. [ Links ]
33. Small, B. J., Dixon, R. A., McArdle, J. J., & Grimm, K. J. (2012). Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria longitudinal study. Neuropsychology, 26, 144-155. [ Links ]
34. Sobral, M., & Paúl, C. (2013). Education, leisure activities and cognitive and functional ability of Alzheimer's disease patients: A follow-up study. Dementia & Neuropsychologia, 7, 181-189. [ Links ]
35. Staff, R. T., Murray, A. D., Deary, I. J., & Whalley, L. J. (2004). What provides cerebral reserve? Brain, 127, 1191-1199. [ Links ]
36. Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015-2028. [ Links ]
37. Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology, 11, 1006-1012. [ Links ]
38. Suo, C., León, I., Brodaty, H., Trollor, J., Wen, W., Sachdev, P., & Valenzuela, M. J. (2012). Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. Neuroimage, 63, 1542-1551. [ Links ]
39. Tamayo, F., Casals-Coll, M., Sánchez-Benavides, G., Quintana, M., Manero, R. M., Rognoni, T., ... Peña-Casanova, J. (2012). Estudios normativos españoles en población adulta joven (Proyecto NEURONORMA Jóvenes): Normas para las pruebas span verbal, span visuoespacial, Letter-Number Sequencing, Trail Making Test y Symbol Digit Modalities Test. Neurología, 27, 319-329. [ Links ]
40. Tucker, A. M., & Stern, Y. (2011). Cognitive reserve in aging. Current Alzheimer Research, 8, 354-360. [ Links ]
41. Valenzuela, M. J., & Sachdev, P. (2007). Assessment of complex mental activity across the lifespan: Development of the Lifetime of Experiences Questionnaire (LEQ). Psychological Medicine, 37, 1015-1025. [ Links ]
42. Valenzuela, M. J., Sachdev, P., Wen, W., Chen, X., & Brodaty, H. (2008). Lifespan mental activity predicts diminished rate of hippocampal atrophy. PEoS One, 3, e2598. doi:10.1371/journal.pone.0002598. [ Links ]
43. Van Dijk, K. R., Van Gerven, P. W., Van Boxtel, M. P., Van der Elst, W., & Jolles, J. (2008). No protective effects of education during normal cognitive aging: Results from the 6-year follow-up of the Maastricht Aging Study. Psychology and Aging, 23, 119-130. [ Links ]
44. Vemuri, P., Lesnick, T. G., Przybelski, S. A., Knopman, D. S., Roberts, R. O., Lowe, V. J., ... Jack, C. R., Jr. (2012). Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Annals of Neurology, 72, 730-738. [ Links ]
45. Wechsler, D. (2004). Escala de Memoria de Wechsler-IH (WMS-III). Madrid: TEA. [ Links ]
46. Wilson, R., Barnes, L., & Bennett, D. (2003). Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology, 25, 634-642. [ Links ]
47. Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Schneider, J. A., & Bennett, D. A. (2013). Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology, 81, 314-321. [ Links ]
48. World Health Organization (2013). Preguntas más frecuentes. Retrieved from http://www.who.int/suggestions/faq/es/. [ Links ]
![]() Correspondence:
Correspondence:
Lola Roldán-Tapia.
Department of Psychology,
Neuroscience Centre,
University of Almeria, s/n,
04120, Almeria (Spain).
E-mail: mdroldan@ual.es
Article received: 13-9-2013
Revised: 30-7-2014
Accepted: 22-9-2014