Introduction
Social anxiety is one of the most prevalent subclinical psychopathological manifestations in teenagers and young adults, and it affects approximately 8-12% of these individuals in the Spanish population (Inglés et al., 2008). In addition, investigations carried out in different countries agree that the clinical presentation of social anxiety is one of the most common disorders worldwide (Brunello et al., 2000).
This clinical entity is characterized by hypersensitivity to criticism or to being viewed negatively by others, as well as discomfort in social situations or social acting, especially in the presence of authority figures, the opposite sex, or strangers. This hypersensitivity occurs both in the presence of real events and in the anticipation of stressful events (Caballo, Arias, Calderero, Salazar and Irurtia, 2011; Brunello et al., 2000; Stein, Torgrud and Walker, 2000).
Several studies have tried to obtain an in-depth understanding of the biological basis for social anxiety. Among them, research on the neuroendocrine response, especially the release of cortisol by the hypothalamus-pituitary-adrenal (HPA) axis, stands out in recent years because cortisol levels are considered a good biomarker of the body's response to stress, and a dysregulation in their secretion can have negative health effects (Almela et al., 2014; Heim, Ehlert and Hellhammer, 2000; Hellhammer, Wüst and Kudielka, 2008; Kirschbaum et al., 1995; Moya-Albiol, Serrano, González-Bono, Rodríguez-Alarcón and Salvador, 2005). Consequently, the HPA axis response to laboratory stressors with a strong social component has been analyzed. However, despite the large amount of literature available, there is no consensus about the existence of a characteristic axis response in people with social anxiety.
On the one hand, an HPA axis hyperresponsiveness to social stress has been found in these subjects, who show a higher cortisol response than non-socially anxious individuals and patients with other anxiety disorders, such as posttraumatic stress disorder (Condren, O'Neill, Ryan, Barret and Thakore, 2002; Roelofs et al., 2009). Moreover, this hyperresponsiveness, compared to non-socially anxious individuals, only occurs when social stressors are employed (Furlan, DeMartinis, Schweizer, Rickels and Lucki, 2001).
However, other studies found no differences in cortisol levels between socially and non-socially anxious individuals in response to a social stressor (Levin et al., 1993; Martel et al., 1999; Espín, Marquina, Hidalgo, Salvador and Gómez-Amor, 2016) on baseline levels (Condren et al., 2002; Potts, Davidson, Krishnan, Doraiswamy and Ritchie, 1991) or after administration of dexamethasone (Uhde, Tancer, Gelernter and Vittone, 1994). In this regard, some authors have suggested that in social anxiety, the cognitive component is predominant, and there is no characteristic physiological response that differentiates individuals with high social anxiety from the rest (Anderson and Hope, 2009; Mauss et al., 2003; Wilhelm et al., 2001). In fact, these individuals tend to show higher subjective anxiety than other people, but a correspondence has not always been found between the physiological response and the psychological response (Beaton et al., 2006; Condren et al., 2002; Furlan et al., 2001; Klumbies, Braeuer, Hoyer and Kirschbaum, 2014; Mauss, Wilhem and Gross, 2003; Roelofs et al., 2009; Wilhelm, Kochar, Roth and Gross, 2001).
On the other hand, there is not enough consistency in the temporal pattern of cortisol secretion in people with social anxiety exposed to stress. Martel et al. (1999) found an increased release of cortisol in the anticipatory or pre-stress phase, and a decrease following the cessation of the stressor. Along these lines, Beaton et al. (2006) found a progressive decrease in cortisol levels from baseline until the stressor ended. Nevertheless, other authors have observed a higher cortisol response during stress (Condren et al., 2002), or in both the stress and post-stress phases (Furlan et al., 2001; Roelofs et al., 2009), compared to previous phases. These findings suggest that the cortisol response is linked mostly to the real presence of the stressor, and not as much to its anticipation, even though the anticipatory anxiety response is a salient clinical characteristic of social anxiety (Clark and Wells, 1995).
It is important to obtain further evidence in order to draw stronger conclusions about the pattern of the psychophysiological stress response in individuals with social anxiety, due to its high prevalence and the discrepancies found in previous studies. Our aim was to determine the salivary cortisol and subjective anxiety responses in high and low socially anxious individuals exposed to an acute stressor. Additionally, a control group of high and low socially anxious individuals not subjected to stress was included, which has not been done in many previous studies. We hypothesized that participants with high social anxiety would show a greater cortisol response and more subjective anxiety under stress than those with low social anxiety, and that these increases in cortisol would occur in the stress and post-stress phases.
Methods
Participants
The sample was composed of twenty-six healthy students (without any neurological, psychiatric, endocrine, or cardiovascular pathology, or drug consumption that could interfere in hormonal levels, such as nicotine or benzodiazepines), obtained by means of non-random sampling and using a cross-sectional design. The presence of a stressful life event during the past six months was also considered an exclusion criterion. With the aim of controlling hormonal changes associated with the menstrual cycle, only women who had consumed oral contraceptives for at least six months were included (Espín et al., 2013; Villada et al., 2014).
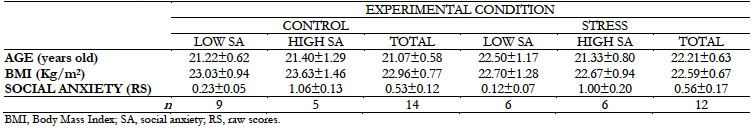
All participants had a medium-high socioeconomic status and were between 18 and 26 years old (21.62 ± 0.43 years old); 58.6% were men and 41.4% women. Their mean Body Mass Index (BMI) was 22.78 ± 0.50 Kg/m2, and scores on social anxiety symptomatology were medium-low in all of them, corresponding to normal scores in the general Spanish population (Table 1) in both sexes. The participants were randomly assigned to two conditions, control or stress, and within each condition, they were subdivided according to their social anxiety level: high or low. Table 1 shows the distribution of the sample and its characteristics.
No statistical differences were found in socioeconomic status, age, BMI, or social anxiety based on gender, experimental condition, or their social anxiety level, both overall and separately for each condition (all p > .05).
All participants provided written informed consent, and the protocol was approved previously by the ethics committee. Once the experiment had ended, they were thanked for their collaboration and given a reward worth 15 euros.
Instruments
Questionnaires
The Hypersensitivity subscale from the Brief Symptom Check List (LSB-50) (De-Rivera and Abuín, 2012), which explores interpersonal and intrapersonal sensitivity and discomfort in social situations or social acting, was used to measure social anxiety. This scale features seven items with a 4-point Likert scale ranging from 0 (not at all) to 4 (very much), and total scores range from 0 to 4, which results from the arithmetic mean of the scores on all the items.
The State subscale from the State-Trait Anxiety Inventory (Spielberger, Gorsuch and Lushene, 1970; adapted to the Spanish population by Seisdedos, 1988) was used to measure subjective anxiety in various phases of the experiment (baseline, stress, and post-stress). It is a self-report measure composed of 20 items rated on a 4-point Likert scale ranging from 0 (not at all) to 3 (extremely). The total scores range between 0 and 60.
Cortisol
Saliva samples were collected using salivettes (Sarstedt, Nümbrecht, Germany) for cortisol in different phases of the experiment (baseline, pre-stress, stress, and post-stress). Participants were instructed to keep the cotton swab in their mouths for 2 min. The samples were centrifuged at 3000 rpm for 15 min, resulting in a clear supernatant with low viscosity that was stored at -20ºC until the analyses were performed. Salivary cortisol concentrations were determined in duplicate with the salivary cortisol enzyme immunoassay kit from Salimetrics (Newmarket, UK). Assay sensitivity was < .007 ug/dL. For each subject, all the samples were analyzed in the same trial. The criterion for measurement replication was fixed as an inter-duplicate. Samples were analyzed in duplicate, and intra-assay and inter-assay coefficients of variation were below 8%. Cortisol levels are expressed in nmol/l.
Procedure
The sessions took place in the afternoon, between 3:00 pm and 5:00 pm. Upon arrival, the participants were asked about their compliance with the recommendations given prior to the sessions (abstain from consuming stimulants or depressive substances, such as tobacco, methylxanthines or alcohol, or eating and drinking in the 2 hours prior to the session, sleep as long as usual, and refrain from any intense physical activity or alcohol consumption the day before the session). They were randomly assigned to a control or stress condition.
This study falls within the context of a larger study that also evaluates cortical activity with an electroencephalogram while viewing emotional stimuli. The entire experimental protocol was divided into five phases: baseline, image visualisation, pre-stress, stress, and post-stress. During the baseline phase (40 min), participants filled in a general questionnaire on sociodemographic data, and later, baseline measurements of subjective anxiety and salivary cortisol were recorded. In the visualization phase (37 min), cortical electrical activity was recorded with an electroencephalogram to display emotional and neutral pictures. Because this part of the experiment is not part of this publication, it will not be discussed in subsequent sections. In the pre-stress phase (10 min), participants were informed about the task to be performed, varying the explanation according to the experimental condition (stress or control), and saliva samples were obtained. The stress phase (10 min) consisted of the application of a standardized laboratory stress test, and subjective anxiety measures and saliva samples were collected. Finally, in the post-stress phase (16 min), these measurements were collected again.
Figure 1 schematically shows the experimental protocol with the duration of each phase relative to the start of the stressor and the collection times of the different saliva samples and subjective anxiety measures.

Figure 1 Study design. Time schedule for collecting saliva samples and measurement of subjective anxiety.
Stress Procedure
The standardized laboratory stress task employed was the Maastricht Acute Stress Test (MAST) (Smeets et al., 2012), which combines physical and psychosocial components, both presented repeatedly and successively. Whereas physical stress is associated with activation of the autonomous nervous system, and less with the release of cortisol (Lovallo, 1975; Smeets et al, 2012), psychosocial stress is associated with increased activation of the HPA axis and, subsequently, with the release of cortisol due to components of social evaluation and uncontrollability (Dickerson and Kemeny, 2004).
The MAST has a stress version and a control adaptation. The stress version assesses the ability to perform a complex arithmetic task with alternating periods of physical stress (introducing one's hand in cold water at 3ºC). With the aim of generating greater psychosocial stress, in the MAST's stress version we placed a video camera in front of them that simulated recording, and the evaluator who applied the test was always someone the participant did not know (not the researcher) and of the opposite sex to the participant.
The control condition was carried out by the researcher, employing an easy arithmetic task (count from 1 to 25) and without feedback or recording, and warm water (36º C) was used as the physical component.
All participants were seated during the application of the test, and the evaluator or the researcher remained standing beside them.
Data analysis
The participants were divided according to their social anxiety (high vs. low), based on percentile 50. Therefore, a balanced division of the sample was ensured, and the risk of tendentiousness in the results was decreased, as might occur by using a split criterion based on extreme scores. ANOVAs for repeated measures (4x2x2) were carried out to evaluate the stress effect on subjective anxiety and cortisol levels, with “experimental condition” (stress vs. control) and “social anxiety” (high vs. low) as between-subjects factors. As within-subject factor, we added cortisol levels measured in the four phases (baseline, pre-stress, stress, and post-stress) and subjective anxiety measured in three phases (baseline, stress, and post-stress). Post hoc Bonferroni's test was used. Greenhouse-Geisser was used when the requirement of sphericity was violated. Delta (Δ) for subjective anxiety and cortisol were calculated for the stress phase (average scores in stress phase minus average scores in baseline phase). The relationship between the Delta (Δ) for cortisol and subjective anxiety was analyzed with Spearman's correlation coefficient, and coefficients obtained by high and low socially anxious participants were compared using Fisher's r-to-z transformation. We used SPSS 20.0 to perform the statistical analyses. A p of < .050 was considered significant.
Results
Stress response
As a starting point, both greater subjective anxiety (F(2, 44) = 20.44, p = .0001, η2 = 0.482) and increased levels of cortisol (F(1.35, 28.29) = 7.11, p = .007, η2 = 0.253) in the stress condition compared to the control condition were verified.
Cortisol levels
The results revealed a higher salivary cortisol response in individuals with high social anxiety than in those with low social anxiety in the stress condition (F(1, 21) = 9.55, p = .006, η2 = 0.313) (Figure 2). Specifically, individuals with high social anxiety showed greater cortisol levels in the stress phase (p = .006) and the post-stress phase (p = .005), than those with low social anxiety. Regarding the control condition, no cortisol differences were observed based on the social anxiety level (F(1, 21) = 0.02, p = .878).
Pattern of cortisol
With regard to the temporal pattern of cortisol levels, participants with high social anxiety under stress showed, in comparison with baseline and pre-stress phases, higher levels in the stress and the post-stress phases (p < .021). No significant differences were found between baseline and the pre-stress phase (p = .072) (Figure 3).
Subjective anxiety
The repeated-measures ANOVA revealed a marginal effect of the social anxiety * experimental condition interaction (F(1, 22) = 3.82, p = .064), so that individuals with high social anxiety showed higher subjective anxiety scores than the low social anxiety individuals when exposed to stress (p = .055) (Figure 4).
Relationships between subjective anxiety and cortisol responses
Finally, Spearman's coefficient correlation between the Δ of subjective anxiety and cortisol under stress was significant for both high (rho = 0.796, p < .010) and low (rho = 0.416, p < .050) social anxiety, and no differences were found between their correlations (z = 1.41, p = .158).
Discussion
The main aim of this research was to compare the neuroendocrine and psychological response to a social stressor depending on the level of social anxiety, as well as determine the pattern of cortisol response at different stress phases and its relation to the measures of subjective anxiety.
As a starting point, both a greater subjective anxiety and increased response of cortisol at stress condition compared to the control condition was verified. Therefore, it can be assumed that the stressor elicited a substantial psychophysiological stress response, confirming the effectiveness of the MAST found in other investigations (Goff, Ali and Pruessner, 2013; Smeets et al., 2012).
In general, participants with high social anxiety have experienced increases in cortisol and subjective anxiety, mainly after application of the stressor, and there has been a correlation between subjective anxiety and salivary cortisol during stress, regardless of the level of social anxiety.
The results have shown an increase in salivary cortisol levels in both high and low socially anxious participants at stress condition, but this response was significantly higher in those with higher social anxiety. This finding would support the idea of increased HPA axis reactivity to social stress in individuals with high social anxiety. Along these lines, Roelofs et al. (2009) measured the cortisol changes in response to the TSST, and they found that individuals with high social anxiety showed higher salivary cortisol secretion than those with low social anxiety. The same results were found by Condren et al. (2002) and Furlan et al. (2001) in a sample of patients diagnosed with social phobia. By contrast, in studies carried out by Levin et al. (1993), Martel et al. (1999), and Espin et al. (2016), no differences were observed in cortisol levels between people with high and low social anxiety when they were subjected to a public speaking task. These discrepancies could be due to the use of a sample of adolescents (Martel et al., 1999), who could have high reactivity to stress across the board, making it difficult to discriminate between high and low social anxiety individuals, or to the use of a laboratory test that may not be stressful enough in this population (Levin et al., 1993; Martel et al., 1999). With regard to Espin et al. (2016), the absence of differences between the two groups could be due to the fact that the stress phase was performed 24 hours after the first visit to the laboratory, which could have produced habituation in the participants.
With regards to the pattern of salivary cortisol response in participants with high social anxiety, anticipatory response has not been observed, but increases during the stress and post-stress phases were seen. Our results agree with other studies that found the same response in clinical populations with social anxiety (Condren et al., 2002; Roelofs et al., 2009). However, some studies have found high levels of cortisol in the anticipatory phase, followed by a decrease after stress (Martel et al., 1999), or even a decrease in both phases, pre-stress and stress, compared to baseline (Beaton et al., 2006). Some authors have reported a bimodal cortisol response pattern, distinguishing between respondent individuals, who have increases after stress, and non-respondents, who experience a decrease (Furlan et al., 2001; Klumbies et al, 2014). The cortisol decrease in some studies could be explained by social anxiety severity, as it has been pointed out that in the most serious cases of psychopathology, lower levels of cortisol are found after stress (Furlan et al., 2001; Heim et al., 2000). However, some studies that reported a decline were performed with healthy people without a serious pathology (Beaton et al., 2006; Martel et al., 1999). Therefore, the influence may be due to additional features, such as other characteristics of the sample, the experimental situation, or the type of stressor employed.
With regard to the sample, individual differences could have an influence, such as the age of the participants, so that at older ages increases in cortisol would be found after stress (Condren et al., 2002; Klimes-Dougan, Hastings, Granger, Usher & Zhan-Waxler, 2001; Martel et al., 1999; Roelofs et al., 2009), or gender, as studies that found a decrease in salivary cortisol levels employed a predominantly female sample (Beaton et al., 2006; Martel et al., 1999). None of these studies controlled the menstrual cycle or the use of oral contraceptives, which are known to influence cortisol levels after stress (Villada et al., 2014; Espin et al., 2013). The cortisol increases observed in the present study, in contrast to others, could be explained by the use of a balanced sample by gender (Roelofs et al., 2009; Condren et al., 2002) and controlled consumption of contraceptives. Moreover, other characteristics of the sample that could influence the results obtained by previous research would be a small sample size or the presence of psychiatric comorbidity, such as depressive symptoms (Yoon and Joormann, 2012).
Regarding the stressor employed in other studies, the lack of a control condition keeps studies from verifying that the test used was a good stress inducer. On the other hand, there is great variety in the stress procedures employed, which makes it difficult to establish the determinant stimulus for each type of response observed. Nonetheless, studies that found post-stress increases in cortisol in socially anxious individuals had the same stressor, which consisted of performing a varied and complex arithmetic task in the presence of multiple unfamiliar observers (Condren et al., 2002; Roelofs et al., 2009). By contrast, decreases in cortisol in this population were associated with performing a prepared speech (Martel et al., 1999; Beaton et al., 2006), and bimodal responses were associated with a prepared speech (Furlan et al., 2001) and a combination of both an arithmetic task and a prepared speech (Klumbies et al., 2014). Therefore, generally, the increases in cortisol in people with social anxiety have been associated with the use of an arithmetic task as the stressor. A particularity of this task, compared to others, lies in the individual's inability to prepare it in advance, in contrast to performing a speech on a topic established minutes earlier. In fact, uncontrollability is a stimulus commonly considered to be a generator of stress in social anxiety (Mattick and Clarke, 1998). It would be consistent with the results and the method used in the present study because an arithmetic task was used as the stressor. Other variables could be influencing our results, such as physical closeness between evaluator and participant (Wilhelm et al., 2001), negative feedback (Vera-Villarroel, Garcia-Lopez and Olivares, 2003), or the presence of an unfamiliar evaluator of the opposite sex (Caballo et al., 2011; Goff et al., 2013).
Finally, the present study also employs a physical stressor, which might facilitate a higher cortisol response. However, several studies have linked the cortisol response primarily to psychosocial stress (Dickerson and Kemeny, 2004; Lovallo, 1975; Smeets et al, 2012), and Furlan et al. (2001) found no differences in the cortisol response of high and low socially anxious individuals under physical stress, but they did under social stress. Thus, the observed differences cannot be attributed to this component of physical stress.
With regards to subjective anxiety, marginally, significant differences were observed, so that individuals with high social anxiety have a higher subjective anxiety than the low social anxiety ones when subjected to stress, which is consistent with previous studies (Klumbies et al., 2014; Mauss et al., 2003; Roelofs et al., 2009; Furlan et al., 2001; Wilhelm et al., 2001). Only Condren et al. (2002) did not achieve to find significant differences, which could be related to the use of a smaller sample.
This finding disagrees with previous research showing that people with social anxiety exhibit a disproportionate subjective anxiety response compared to their cortisol release (Furlan et al., 2001; Klumbies et al., 2014) or autonomic reactivity (Anderson and Hope, 2009; Mauss et al., 2003; Wilhelm et al., 2001). Previous discrepancies between subjective anxiety and cortisol measures agree with the presence of non-cortisol-responders. However, none of these studies performed correlations between the two measures in people with social anxiety (Klumbies et al., 2014; Furlan et al., 2001). The present study found not only that there are differences in both measures between high and low socially anxious individuals, but also a significant correlation between them.
In any case, this research has certain limitations, such as the small sample size. However, despite this limitation, we have been able to observe statistically significant differences between groups and a higher proportion of cortisol respondents among those with high social anxiety (4 out of 6), compared to those with low social anxiety (1 out of 6), under stress, which gives added value to the results obtained. On the other hand, due to our interest in studying healthy populations, another limitation could be the inability to generalize the results to a clinical population.
In future research, it would be interesting to determine the role played by the hypersecretion of cortisol in the state of physical and/or mental health of individuals with subclinical social anxiety. Furthermore, research in contexts with greater ecological validity could provide additional evidence about the temporal pattern of cortisol manifested by these individuals in their everyday lives.
Conclusions
Our findings suggest the existence of a deregulated HPA axis response to stress in young people with subclinical social anxiety compared to individuals with less social anxiety, which warns us about the possible impact this could have on health in this population (Lundberg, 2005; McEwen, 1998; Sapolsky, 1996). Moreover, the stress response in individuals with social anxiety would be mediated by different characteristics of the experimental situation, with uncontrollability experienced while performing the task being one of the characteristics that may play a predominant role (Dickerson & Kemeny, 2004).
Due to the presence of a differential response to these kinds of tasks depending on the level of social anxiety, research that includes them in their experimental protocols should measure social anxiety because it can influence the results. Indeed, even though our sample groups did not show extreme mean scores on the social anxiety subscale used, their cortisol responses differed substantially during stress.