Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
The European Journal of Psychiatry
versión impresa ISSN 0213-6163
Eur. J. Psychiat. vol.24 no.2 Zaragoza abr./jun. 2010
Do depressive symptoms correlate with oxidative stress in a sample of healthy college students?
Masateru Matsushita*,**,***; Takayuki Kumano-Go*,**,***; Nakamori Suganuma*,**,***; Hiroyoshi Adachi*,**,***; Schubei Yamanura*,***; Hiroko Morishima*,***; Yoshihisa Shigedo*,***; Akira Mikami*,**,***; Masatoshi Takeda*,***; Yoshiro Sugita*,**,***
* Department of Psychiatry, Osaka University Graduate School of Medicine, Suita
** Osaka University Health Care Center, Osaka, Toyonaka
*** Sleep Medical Center, Osaka University Hospital, Suita. Japan
This study was supported by a Grant-in-aid for Young Scientists (B), (No. 18700552) from the Ministry of Education, Science, Sports and Culture of Japan.
ABSTRACT
Background and Objectives: Major depression and sub-threshold depressive symptoms are associated with health crisis. Oxidative stress may be a mechanism for major depression. In the present study, we examined the relationship between the degree of depressive symptoms and oxidative status using a reliable and inexpensive method that evaluates endogenous hydroperoxides.
Methods: We conducted a cross-sectional study in 54 non-smoking college students and measured serum reactive oxygen metabolites (ROMs) and the biological antioxidant potential (BAP) as an index of oxidative status. Depressive symptoms were assessed by the Beck Depression Inventory (BDI).
Results: The concentrations of ROMs did not differ between the lower BDI group (BDI < 14) and the higher BDI group (BDI > 14) (282.7 - 59.84 U.CARR vs 307.7 - 67.51 U.CARR, z = -1.19, P = 0.239). We did find a significant relationship between ROM concentration values and higher BDI scores (rho = 0.30, P = 0.042). BAP levels in the hig-her BDI group were not significantly greater than those in the lower BDI group (z = -0.108, P = 0.287). There was no significant correlation between BAP and depressive symptoms (rho = 0.22, P = 0.140). Moreover, we conducted a multiple regression analysis to control for gender difference and difference in sleep perception of the previous night between the two BDI groups. However, depressive symptoms were not significantly predicted by ROM concentrations (b = 0.28, P = 0.076).
Conclusions: While results of the present study demonstrated a slight correlation between depressive symptoms and oxidative stress, this linkage could not be confirmed after controlling for significant confounding factors. This result should be verified in a larger sample.
Key words: Depression; Depressive symptoms; Oxidative stress.
Introduction
Major depression is serious public health problems that increase the risk of comorbid diseases such as cardiovascular disease1-3, stroke4, cancer5-7 and diabetes8-10. Oxidative stress has gained attention in psychiatric medicine, and various markers of oxidative stress have been identified11. Recently, reactive oxygen metabolites (ROMs) and the biological antioxidant potential (BAP) were used to evaluate oxidative status, and their significance as clinical markers has been reported in various medical fields12-15. Although the increase in lipid peroxidation products and elevated oxidative DNA da-mage have been demonstrated in depressed subjects16-18, the ROMs and BAP have not been examined. We, therefore, aimed to exa-mine the relationship between depressive symptoms and oxidative stress, using the ROMs and BAP.
Methods
Participants and procedure
Participants were 54 non-smoking students. These volunteers were excluded from analysis if they reported a history of psychiatric disorders, an irregular sleep-wake rhythm, taking a supplement, or drinking alcoholic beverages almost daily. None of those included in the analysis had sought medical care in the month previous to the study. Female participants were excluded from analysis if they were menstruating. Forty-seven participants were included in the data analysis.
We asked participants to maintain regular hours for bedtime and awakening, and to abstain from eating and drinking except for water and tea after the usual dinner hour. On the following day between 9:00 am and 12:00 noon we obtained a fasting blood sample and assessed depressive symptoms.
Subjects were asked to complete self-administered questionnaires, and answered items on the lifestyle as well as other characteristics such as age, gender, menstrual state, height and weight, and history of psychiatric disorders. Depressive symptoms were assessed by the Beck Depression Inventory-II (BDI), which consists of 21 items. Scores were scaled from 0 to 63, with higher scores indicating greater depressive symptoms. Total scores can be classified as indicating four severity categories: minimal (0-13), mild (14-19), moderate (20-28), and severe (29-63)19. Participants were classified into two groups according to BDI scores: a lower BDI group (0-13) and a higher BDI group (14-39). Questions were also asked regarding personality and anxiety. These findings are to be reported in a separate paper.
Assay of oxidative status
ROMs were evaluated by the d-ROMs test (Diacron International, Grosseto, Italy) as an index of oxidative stress, which estimates endogenous hydroperoxide. The 10ml of Blood was collected from all participants by finger puncture with disposable lancets and analyzed with the free radical analysis system (FRAS) immediately after collection. The results of the d-ROM test are expressed in arbitrary units called "Carratelli Units" (U.CARR).
The BAP test was performed by the FRAS system, which includes both a photometric device and an incorporated thermostatic mini-centrifuge. This test measures the blood concentration of antioxidants as agents able to reduce iron from its ferric (Fe3+) to ferrous form (Fe2+). We estimated the intensity of this chromatic change photometrically. The results are expressed as mmol/l.
Statistical analysis
In this study, we used the Mann-Whitney U test, chi-square test, and Spearman´s correlation analysis. Additionally, if we found a significant linkage, multiple regression analysis was applied to assess the association of the depressive symptoms with potential confounding factors.
Results
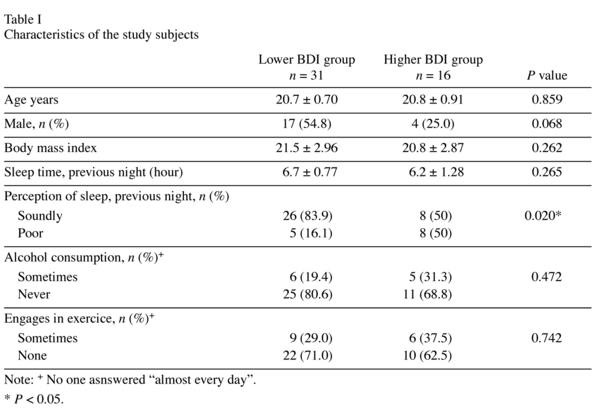
The demographic data and results are summarized in Table I. There was a difference in perception of the previous night´s sleep. Regarding the possible effects of gender differences in results of assessment of ROMs and BAP, female students had a hig-her value for ROMs compared to males (z = -3.18, P = 0.001), but there was no difference in the BAP value (z = -0.77, P = 0.438).
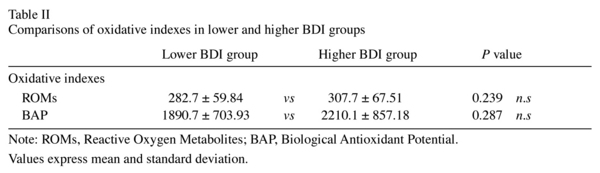
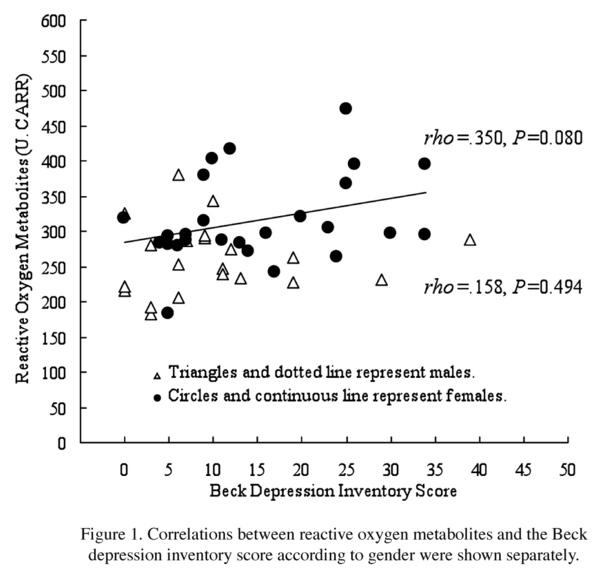
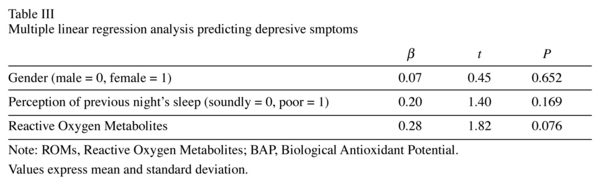
Participants in the higher BDI group had a mean ROM level of 307.7 ± 67.51 U.CARR and those in the lower BDI group had a mean ROM level of 282.7 ± 59.84 U.CARR (Table II), with no significant difference between the two groups. Likewise, the mean BAP level in the higher BDI group was not significantly higher than that in the lower group, and no significant correlation was observed between the BDI score and BAP (rho = 0.22, P = 0.140). However, we found a weak correlation between the BDI score and the ROM concentration (rho = 0.30, P = 0.042). Figure 1 shows a scatter plot of the relationship between oxidative stress and depressive symptoms. Only in female participants there was a statistically slight relationship between depressive symptoms and ROMs. Because between-groups differences in gender and the perception of sleep were found, we applied multivariate analysis to control the effect for of these differences statistically. As shown in Table III, although the BDI score appeared to have a relationship with the ROM value after control for the confounding factors, the probability did not reach the 5% level of statistical significance.
Conclusions
In this study, we found that depressive symptoms had a slight correlation with the increased concentration of ROMs, although no significant differences in the levels of ROMs and BAP between the two BDI groups were observed. Previous studies have demonstrated a significant correlation between oxidative stress and depression16-18, 20, 21. Furthermore, Bilici et al. reported that lipid peroxidation and antioxidative enzyme levels were significantly decreased to normal levels after selective serotonin reuptake inhibitor therapy for 12 weeks22. However, our results showed no clear correlation between depressive symptoms and oxidative status. This may be due to the relatively mild depressive symptoms in our subjects.
There is a possible mechanism that links the oxidative state with depressive symptoms. Hypothalamic-pituitary-adrenal (HPA) axis overactivity is frequently observed in those with major depression23. A HPA axis stimulated by psychological stress may enhance the production of reactive oxygen species relevant to cytotoxicity24. Moreover, depression has been related to increased immune activity. Leukocytes, monocytes, T helper cells, and interleukin are immune inflammatory markers that are increased in depressive disorders25-28.
The d-ROMs and BAP tests, which are minimally invasive, easy and inexpensive, provide information on the general wellness state of the body for the clinician. To our knowledge, this study is the first attempt to examine an association between depressive symptoms and ROMs. Additional studies that include a larger number of participants and subjects with major depression disorders are needed.
References
1. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55(7): 580-592. [ Links ]
2. Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry 2003; 54(3): 227-240. [ Links ]
3. Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66(3): 305-315. [ Links ]
4. Larson SL, Owens PL, Ford D, Eaton W. Depressive disorder, dysthymia, and risk of stroke: thirteen-year follow-up from the Baltimore epidemiologic catchment area study. Stroke 2001; 32(9): 1979-1983. [ Links ]
5. Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst 1998; 90(24): 1888-1893. [ Links ]
6. Raison CL, Miller AH. Depression and cancer: new developments regarding diagnosis and treatment. Biol Psychiatry 2003; 54(3): 283-294. [ Links ]
7. Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry 2003; 54(3): 269-282. [ Links ]
8. Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes type 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry 2003; 54(3): 317-329. [ Links ]
9. Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res 2002; 53(4): 903-906. [ Links ]
10. Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care 1996; 19(10): 1097-1102. [ Links ]
11. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008; 11(6): 851-876. [ Links ]
12. Atabek ME, Vatansev H, Erkul I. Oxidative stress in childhood obesity. J Pediatr Endocrinol Metab 2004; 17(8): 1063-1068. [ Links ]
13. Digiesi V, Oliviero C, Giannò V, Rossetti M, Fiorillo C, Oradei A, et al. Reactive metabolites of oxygen, lipid peroxidation, total antioxidant capacity and vitamin E in essential arterial hypertension. Clin Ter 1997; 148(11): 515-519. [ Links ]
14. Katsabeki-Katsafli A, Kerenidi T, Kostikas K, Dalaveris E, Kiropoulos TS, Gogou E, et al. Serum vascular endothelial growth factor is related to systemic oxidative stress in patients with lung cancer. Lung Cancer 2008; 60(2): 271-276. [ Links ]
15. Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, et al. A simple test to monitor oxidative stress. Int Angiol 1999; 18(2): 127-130. [ Links ]
16. Yanik M, Erel O, Kati M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatrica 2004; 16(4): 200-203. [ Links ]
17. Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res 2005; 39(6): 553-560. [ Links ]
18. Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun 2003; 331(4): 1014-1018. [ Links ]
19. Kojima M, Furukawa TA, Takahashi H, Kawai M, Nagaya T, Tokudome S. Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res 2002; 110(3): 291-299. [ Links ]
20. Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2´-deoxyguanosine in clinical depression. Psychosom Med 2006; 68(1): 1-7. [ Links ]
21. Tsuboi H, Shimoi K, Kinae N, Oguni I, Hori R, Kobayashi F. Depressive symptoms are independently correlated with lipid peroxidation in a female population: comparison with vitamins and carotenoids. J Psychosom Res 2004; 56(1): 53-58. [ Links ]
22. Bilici M, Efe H, Köroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 2001; 64(1): 43-51. [ Links ]
23. Ansseau M. Diagnostice and therapeutic usefulness of biological markers of depression. University of Liège, Thèse d´ Agrégation; 1992. [ Links ]
24. Kasckow JW, Baker D, Geracioti TD Jr. Cortico-tropin-releasing hormone in depression and post-traumatic stress disorder. Peptides 2001; 22(5): 845-851. [ Links ]
25. Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, et al. Evidence for a systemic immune activation during depression, results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med 1992; 22(1): 45-53. [ Links ]
26. Deger O, Bekaroglu M, Orem A, Orem S, Uluutku N, Soylu C. Polymorphonuclear (PMN) elastase levels in depressive disorders. Biol Psychiatry 1996; 39(5): 357-363. [ Links ]
27. Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Increased CD56+ natural killer cells and related cytokines in major depression. Clin Immunol Immunopathol 1996; 78(1): 83-85. [ Links ]
28. Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, et al. Indicators of immune activation in major depression. Psychiatry Res 1996; 64(3): 161-167. [ Links ]
![]() Correspondence:
Correspondence:
Masateru Matsushita
Psychiatry Department of Integrated Medicine
Division of Internal Medicine
Osaka University Graduate School of Medicine
2-2-D3 Yamadaoka, Suita
Japan 565-0871
Tel.: +81-6-6879-3055
Fax: +81-6-6879-3059
E-mail: matsushit@psy.med.osaka-u.ac.jp
Received: 22 December 2008
Revised: 20 December 2009
Accepted: 4 February 2010