Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
The European Journal of Psychiatry
versión impresa ISSN 0213-6163
Eur. J. Psychiat. vol.30 no.2 Zaragoza abr./jun. 2016
Alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal
Mehmet Bülent Sönmeza; Yasemin Görgülüa; Rugul Köse Çınara; Evnur Kahyacı Kılıçb; Aycan Ünalc and Mehmet Erdal Vardard
a Assistant Professor, MD, Trakya University, School of Medicine, Department of Psychiatry, Edirne. Turkey
b MD, Trakya University, School of Medicine, Department of Psychiatry, Edirne. Turkey
c Biologist, Trakya University, School of Medicine, Department of Medical Biochemistry, Edirne. Turkey
d Professor, MD, Trakya University, School of Medicine, Department of Psychiatry, Edirne. Turkey
This study was supported by grant form the Scientific Research Projects Unit of the Trakya University, Edirne, Turkey (Project No: 2013-90).
ABSTRACT
Background and Objectives: Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are neurotrophic neuropeptides that play important roles in the synaptic plasticity, neuronal growth, survival and function. A possible neuroprotective role of neurotrophic factors against alcohol-induced cell damage has been suggested, and dysregulations in neurotrophic factors may be involved in the vulnerability to addiction. The aim of this study was to investigate the alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal compared to healthy controls.
Methods: BDNF and GDNF serum levels of 34 male inpatients diagnosed with alcohol addiction according to DSM-IV-TR were investigated during alcohol withdrawal (day 1, 7 and 14) in comparison to 32 healthy controls using an enzyme-linked immunosorbent assay (ELISA). Severity of alcohol withdrawal was measured by Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar), and intensity of alcohol craving was measured by Penn Alcohol Craving Scale (PACS) during alcohol withdrawal (day 1, 7 and 14).
Results: BDNF serum levels increased significantly during alcohol withdrawal (p = 0.020). They were negatively correlated to the severity of alcohol withdrawal, and the correlation was close to being statistically significant (p = 0.058). BDNF and GDNF serum levels did not differ significantly between the patient and control groups. GDNF serum levels did not change significantly during alcohol withdrawal.
Conclusions: Our results may provide support for the previously hypothesized role of BDNF in the neuroadaptation during alcohol withdrawal.
Keywords: BDNF; GDNF; Alcohol; Addiction; Withdrawal.
Introduction
Alcohol addiction is a chronic relapsing disorder characterized by repetitive and uncontrolled alcohol drinking patterns. Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are neurotrophic neuropeptides that play important roles in the synaptic plasticity, neuronal growth, survival and function1-3. A possible neuroprotective role of neurotrophic factors against alcohol-induced cell damage has been suggested, and dysregulations in neurotrophic factors may be involved in the vulnerability to addiction and in the brain damage caused by long-term alcohol exposure1-7.
Animal studies have shown that acute alcohol consumption results in increased BDNF expression, whereas prolonged alcohol consumption is associated with decreased BDNF expression8,9. Acute releases in BDNF during initial exposure to alcohol tends to degrade during chronic alcohol consumption, which may increase the severity of withdrawal symptoms8,10,11. Moreover, it has been reported that increased behavioral responses to alcohol in rats is associated with decreased BDNF expression9. BDNF is one of the most abundant neurotrophic factors expressed in the central nervous system, and it is also present in human peripheral blood7,12. Clinical studies have reported contradictory results including decreased, increased and nonaltered BDNF serum levels of alcohol-addicted patients1,3,5,7,13-15. Recent studies have suggested the association between acute withdrawal severity and BDNF serum levels3,16,17.
GDNF is an essential growth factor for the development, survival of midbrain dopamine neurons, and it may play a neuroprotective role of neurotrophic factors against alcohol-induced cell damage2,18. Animal studies have suggested that GDNF may be a negative regulator of biochemical and behavioral adaptations in alcohol addiction, and it suppresses self-administration of alcohol18-20. There are a few clinical study results that show alterations of GDNF serum levels in alcohol addiction or abuse. It has been reported that GDNF serum levels are reduced during chronic alcohol consumption and withdrawal period3, and young individuals with alcohol abuse have increased GDNF serum levels6.
To validate recent study results and to better clarify the putative dysregulations of neurotrophic factors in alcohol addiction, we investigated the alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal compared to healthy controls and the possible associations between BDNF and GDNF serum levels and clinical features related to drinking behavior.
Materials and methods
The present study was a part of the research project (Evaluation of the levels of biochemical markers and neurotrophic factors in patients with alcohol use disorder) approved by the local Ethics Committee of the Trakya University Faculty of Medicine (Project No: 2012-116). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. We investigated BDNF and GDNF serum levels of 34 male inpatients who were diagnosed with alcohol addiction according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) and were admitted for detoxification treatment from June 2013 to September 2014 (the Alcohol and Substance Addiction Treatment and Rehabilitation Center, Trakya University Faculty of Medicine, Edirne, Turkey). Exclusion criteria were axis one psychiatric diagnoses apart from alcohol and nicotine use disorders according to the DSM-IV-TR, delirium tremens, severe neurological diseases such as epilepsy and cerebrovascular diseases, significant physical illnesses such as cardiovascular, hepatic and renal diseases, and intake of psychopharmacological medication.
We assessed the patients with an initial clinical interview to ascertain their DSM-IV-TR diagnoses. All patients underwent a detailed physical examination, routine laboratory testing and urine drug screening. Alcohol consumption was stopped immediately and completely at admission. Patients were treated by various doses of diazepam, which were adjusted by the severity of their withdrawal symptoms and tapered gradually during alcohol withdrawal. The cumulative benzodiazepine dosage used during detoxification was recorded and documented as milligrams of diazepam (minimum: 115 mg, maximum: 740 mg, mean: 318.09 ± 161.49 mg). Oral vitamin B complex (including B1, B6, B12) supplementation were also given to every participant. There was no further psychopharmacological treatment during alcohol withdrawal. Measurements of breath alcohol concentration were performed on admission and during alcohol withdrawal using the alcohol breath analyzer.
The control group included 32 healthy male subjects without known psychiatric and physical illnesses and with normal results for routine laboratory tests. Controls were assessed with an initial clinical interview and screened with the Alcohol Use Disorder Identification Test (AUDIT)21,22. They were negative for alcohol use disorders and any other axis one diagnosis according to DSM-IV-TR. A score below 7 points in the AUDIT was required for inclusion in the control group. They were not receiving any therapeutic treatment.
BDNF and GDNF serum levels were investigated on the next morning of admission for detoxification (day 1), day 7 and day 14 of alcohol withdrawal and were compared to the serum levels of the healthy control group. Fasting blood samples were taken on admission between 8 and 10 am. All blood samples were centrifuged and stored at -80 oC immediately after collection until they were studied. BDNF and GDNF serum levels were assessed using the enzyme-linked immunosorbent assay (ELISA) kits (Boster Biological Technologies, CA, USA). All the assays were performed according to the manufacturer's directions. The BDNF and GDNF values were defined as ng/ml and pg/ml, respectively. Determination of further blood parameters (including aspartate transaminase [AST], alanine transaminase [ALT], gamma-glutamyl transferase [GGT] and complete blood count) was performed using routine clinical laboratory methods.
Additional data (such as sociodemographic data, body mass index [BMI], history of alcohol drinking and smoking behavior) were obtained in a structured interview. Intensity of alcohol craving was measured by the Penn Alcohol Craving Scale (PACS)23,24. Severity of alcohol withdrawal was measured by the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar)25. These measurements were taken once a day on day 1, day 7 and day 14.
Statistical analysis
Descriptive statistics for continuous variables were shown as mean ± standard deviation (SD), categorical variables were expressed as number of cases (n) and (%). According to the normality of distribution, group differences were investigated using the Student's t-test or the Mann Whitney U test, and alterations of the serum levels during alcohol withdrawal were assessed using the one-way analysis of variance (ANOVA) with repeated measures or the Friedman test. Categorical variables were evaluated by the Pearson's Chi square test. Correlations between the BDNF and GDNF levels and other variables were analyzed by the Pearson's correlation coefficient or the Spearman's rank correlation coefficient. Analyses were carried out using the statistical package for the social sciences (SPSS) version 20. Statistical significance was defined as a two-sided p value of < 0.05.
Results
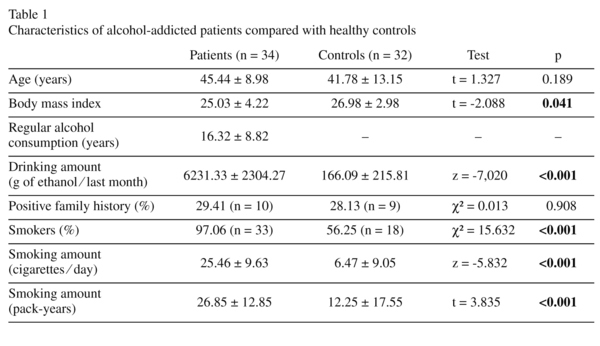
The mean age ± SD and the age range of the alcohol-addicted patients were 45.44 ± 8.98 years and 28 to 61 years, respectively, and no difference was observed from those of the control group (41.78 ± 13.15 years and 27 to 64 years). Characteristics of the alcohol-addicted patients compared with the healthy controls are shown in table 1. There were significant differences between the two groups with regard to BMI, average alcohol consumption amount in the past month, smoker percentage and smoking amount. 29.41% of the patients and 28.13% of the controls reported a positive family history of alcohol addiction. BDNF and GDNF serum levels of the alcohol-addicted patients were not significantly correlated with age (Pearson's r = -0.186, p = 0.291 [BDNF, day 1]; r = 0.195, p = 0.269 [GDNF, day 1]), BMI (r = 0.167, p = 0.396 [BDNF, day 1]; r = -0.147, p = 0.446 [GDNF, day 1]), duration of regular alcohol consumption (r = 0.056, p = 0.755 [BDNF, day 1]; r = 0.250, p = 0.154 [GDNF, day 1]), drinking amount in the past month (r = -0.160, p = 0.366 [BDNF, day 1]; r = -0.056, p = 0.755 [GDNF, day 1]), smoking amount (cigarettes⁄day) (Spearman's rho = 0.029, p = 0.874 [BDNF, day 1]; rho = 0.021, p = 0.908 [GDNF, day 1]) and smoking amount (pack-years) (r = -0.194, p = 0.280 [BDNF, day 1]; r = -0.138, p = 0.445 [GDNF, day 1]).
BDNF and GDNF serum levels of alcohol-addicted patients and healthy controls
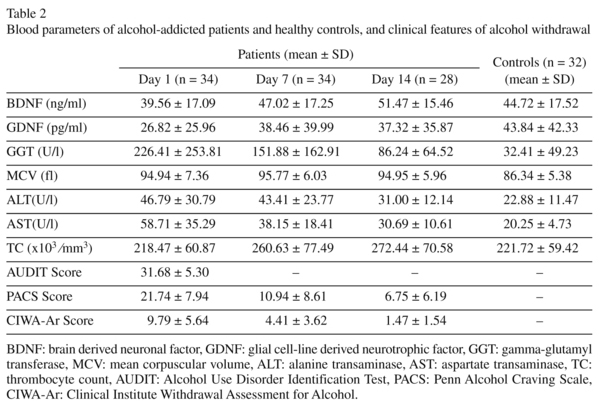
Blood parameters of alcohol-addicted patients and healthy controls, and clinical features of alcohol withdrawal are shown in table 2. BDNF serum levels of the patient group did not differ significantly from BDNF serum levels of the healthy control group (t = -1.212, p = 0.230 [day 1]; t = 0.538, p = 0.592 [day 7]; t = 1.578, p = 0.121 [day 14]). BDNF serum levels increased significantly during alcohol withdrawal (F = 4.221, p = 0.020). In pairwise comparisons, the BNDF serum levels on day 14 were significantly higher than those on day 1 (p = 0.016). There was no significant difference in other pairwise comparisons (between day 1 and day 7, p = 0.363; between day 7 and day 14, p = 0.677).
GDNF serum levels of the patient group did not differ significantly from GDNF serum levels of the healthy control group (z = -1.585, p = 0.113 [day 1]; z = -0.436, p = 0.663 [day 7]; z = -0.630, p = 0.529 [day 14]). GDNF serum levels did not change significantly during alcohol withdrawal (χ2; = 0.500, p = 0.779). BDNF and GDNF serum levels of the alcohol-addicted patients during alcohol withdrawal compared with the healthy controls are shown in figure 1.
Correlations between BDNF, GDNF and other variables in alcohol-addicted patients
BDNF serum levels were significantly correlated with thrombocyte count (TC) (r = 0.494, p = 0.004 [day 1]) and negatively correlated with GGT (rho = -0.402, p = 0.022 [day 1]). BDNF was not significantly correlated with GDNF (r = 0.364, p = 0.480 [day 1]), MCV (r = -0.122, p = 0.500 [day 1]), ALT (r = 0.229, p = 0.193 [day 1]) and AST (r = -0.065, p = 0.716 [day 1]).
GDNF serum levels were not significantly correlated with GGT (rho = -0.140, p = 0.445 [day 1]), MCV (r = -0.086, p = 0.636 [day 1]), ALT (r = 0.028, p = 0.873 [day 1]), AST (r = -0.169, p = 0.340 [day 1]) and TC (r = -0.015, p = 0.932 [day 1]).
BDNF serum levels were negatively correlated to the severity of alcohol withdrawal measured by the CIWA-Ar, and the correlation was close to being statistically significant (r = -0.329, p = 0.058 [day 1]). BDNF was not significantly correlated with AUDIT scores (r = 0.049, p = 0.784 [day 1]) and PACS scores (r = -0.236, p = 0.179 [day 1]).
GDNF serum levels were not significantly correlated with AUDIT scores (r = -0.129, p = 0.468 [day 1]), CIWA-Ar scores (r = -0.114, p = 0.521 [day 1]) and PACS scores (r = -0.212, p = 0.228 [day 1]). BDNF and GDNF serum levels were not significantly correlated with the cumulative benzodiazepine dosage used during alcohol withdrawal (r = -0.083, p = 0.641 [BDNF, day 1]; r = 0.084, p = 0.636 [GDNF, day 1]).
BDNF and GDNF serum levels were not significantly correlated with the futher blood parameters (GGT, MCV, ALT, AST and TC), CIWA-Ar and PACS scores on day 7 and day 14 (data not shown).
Discussion
To the best of our knowledge, this is the second clinical study to investigate the alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal compared to healthy controls and the possible associations between these serum levels and clinical features related to drinking behavior. Our main findings are as follows: (a) BDNF and GDNF serum levels did not differ significantly between the patient and control groups; (b) BDNF serum levels increased significantly during alcohol withdrawal; (c) BDNF serum levels were negatively correlated to the severity of alcohol withdrawal, and the correlation was nearly statistically significant; and (d) GDNF serum levels did not change significantly during alcohol withdrawal.
Preclinical studies have shown that acute alcohol consumption results in increased BDNF expression, whereas prolonged alcohol consumption is associated with decreased BDNF expression8,9. The acute release of BDNF during initial exposure to alcohol tends to degrade during chronic alcohol consumption, which may increase the severity of withdrawal symptoms8,10,11. Preclinical studies have suggested that GDNF may be a negative regulator of biochemical and behavioral adaptations in alcohol addiction, and it suppresses the self-administration of alcohol18-20. Neurotrophic factors may exert a possible neuroprotective role against alcohol consumption and neurotoxicity in the early stages, and their levels and protective effects may decrease along with the development of alcohol addiction3,6.
Our GDNF-related results are contrary to those of a previous clinical study, which investigated the alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal compared to healthy controls. Heberlein, et al3 reported reduced GDNF serum levels during chronic alcohol consumption and the withdrawal period and a negative association of its serum levels with alcohol tolerance. These findings supported the opinion that reduced GDNF serum levels may indicate the reduced capability of neuronal repair of neuronal damage, as is frequently observed in alcohol-addicted patients3. Our results do not confirm this assumed role of the GDNF serum levels. Because the knowledge about peripheral GDNF levels in alcohol-addicted patients is limited, further studies are warranted to better clarify the alterations in GDNF serum levels during acute/chronic alcohol consumption and the withdrawal period.
In the literature, there are studies that report lower peripheral BDNF levels in alcohol-addicted patients5,7,13,26 along with studies that report similar levels to those in healthy controls1,3,14,16. We found no significant alterations between the BDNF serum levels of the patient and control groups. Our results are consistent with previous clinical studies that reported that BDNF levels increase during abstinence1,15,16,26; however, some studies indicate no change in serum BDNF levels during alcohol withdrawal3 or a tendency to decrease in the early stages of abstinence13.
The differences between the results obtained in these studies may be partially explained by different sample sizes, clinically heterogeneous cohorts of patients, different strategies of alcohol detoxification treatment, and genetic differences in the BDNF system3,27. We investigated BDNF and GDNF serum levels in a sample limited to male inpatients that included small number of participants. BDNF and GDNF serum levels did not differ significantly between the patient and control groups, even though the male inpatients might be in a relatively severe stage of alcohol addiction. This might be due to the limited number of subjects. Patients in our study were treated by various doses of diazepam, which were adjusted by the severity of their withdrawal symptoms and tapered gradually during alcohol withdrawal. BDNF and GDNF serum levels were not correlated with the cumulative benzodiazepine dosage, but we could not rule out the effect of the medication used in alcohol detoxification. We investigated the alterations of BDNF and GDNF levels for 2 weeks among the patients undergoing withdrawal. It has been reported that patients abstaining for at least 30 days have lower BDNF levels than controls5, and patients who remained abstinent during the 6 months following alcohol detoxification have higher BDNF levels than non-abstinent patients and controls1. The difference between patients and controls with regard to smoker percentage and smoking amount could have confounded the results. Although BDNF and GDNF serum levels were not correlated with the degree of smoking (cigarettes/day and pack-years), we could not rule out the potential effect of smoking on neurotrophin levels. The difference with regard to BMI between patients and controls could influence the results, even though BDNF and GDNF serum levels were not correlated with BMI. Furthermore, the psychological stress associated with the hospitalization for detoxification treatment may affect BDNF and GDNF serum levels, and measuring concomitant cortisol levels as a possible stress indicator could be helpful to clarify this issue7,28.
Recent studies have suggested the association between serum BDNF levels and acute withdrawal severity3,16,17. In our study, BDNF serum levels were negatively correlated with the severity of alcohol withdrawal measured by the CIWA-Ar, and the correlation was close to being statistically significant. Moreover, BDNF serum levels were significantly correlated with TC and negatively correlated with GGT. It has been reported that circulating BDNF is released by thrombocytes29. While we observed a tendency to increase in TC during alcohol withdrawal, BDNF serum levels were not significantly correlated with TC on day 7 and day 14. TC variations may contribute to the differences in BDNF serum levels, but it is widely accepted that peripheral BDNF levels reflect the BDNF levels in the brain30-32. We observed a decreasing tendency in GGT levels during alcohol withdrawal, but BDNF serum levels were not significantly correlated with GGT on day 7 and day 14. BDNF expression is also detectable in the liver, which is often damaged during chronic alcohol consumption33. If the increased BDNF levels during alcohol withdrawal were caused by liver regeneration, they should have correlated with GGT levels1.
In conclusion, the significant increase of BDNF serum levels during alcohol withdrawal and the negative correlation of these serum levels with the severity of alcohol withdrawal may provide support for the previously hypothesized role of BDNF in neuroadaptation during alcohol withdrawal. Our results do not support the opinion that reduced GDNF serum levels may indicate the reduced capability of neuronal repair of neuronal damage, as is frequently observed in alcohol-addicted patients. The small number of participants used to generate the results of the study is a severe limitation of this study. Further studies including larger samples and the use of a standardized medication schedule during alcohol withdrawal are required to better determine the profile of neurotrophic factors in different stages of alcohol addiction and during early/late periods of alcohol abstinence.
Acknowledgments
The authors thank Prof Hakan Erba from the Department of Medical Biochemistry (Trakya University, School of Medicine, Edirne, Turkey) for laboratory assistance.
References
1. Costa MA, Girard M, Dalmay F, Malauzat D. Brain-derived neurotrophic factor serum levels in alcohol-dependent subjects 6 months after alcohol withdrawal.Alcohol Clin Exp Res. 2011; 35(11): 1966-73. [ Links ]
2. Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010; 35(2): 157-71. [ Links ]
3. Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Gröschl, et al. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34(6): 1060-4. [ Links ]
4. He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005; 25: 619-28. [ Links ]
5. Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007; 31(11): 1833-8. [ Links ]
6. Lhullier AC, Moreira FP, da Silva RA, Marques MB, Bittencourt G, Pinheiro RT, et al. Increased serum neurotrophin levels related to alcohol use disorder in a young population sample. Alcohol Clin Exp Res. 2015; 39(1): 30-3. [ Links ]
7. Zanardini R, Fontana A, Pagano R, Mazzaro E, Bergamasco F, Romagnosi G, et al. Alterations of brain-derived neurotrophic factor serum levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2011; 35(8): 1529-33. [ Links ]
8. Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009; 109(5): 1459-68. [ Links ]
9. McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004; 24(46): 10542-52. [ Links ]
10. Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009; 29(43): 13494-502. [ Links ]
11. Ting-A-Kee R, Vargas-Perez H, Bufalino MR, Bahi A, Dreyer JL, Tyndale RF, et al. Infusion of brain-derived neurotrophic factor into the ventral tegmental area switches the substrates mediating ethanol motivation. Eur J Neurosci. 2013; 37(6): 996-1003. [ Links ]
12. Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003; 4(4): 299-309. [ Links ]
13. Cavus SY, Dilbaz N, Darcin AE, Eren F, Kaya H, Kaya O. Alterations in serum BDNF levels in early alcohol withdrawal and comparison with healthy controls. Bulletin of Clinical Psychopharmacology. 2012; 22(3): 210-5. [ Links ]
14. D'Sa C, Dileone RJ, Anderson GM, Sinha R. Serum and plasma brain-derived neurotrophic factor (BDNF) in abstinent alcoholics and social drinkers. Alcohol. 2012; 46(3): 253-9. [ Links ]
15. Lee BC, Choi IG, Kim YK, Ham BJ, Yang BH, Roh S, et al. Relation between plasma brain-derived neurotrophic factor and nerve growth factor in the male patients with alcohol dependence. Alcohol. 2009; 43(4): 265-9. [ Links ]
16. Huang MC, Chen CH, Chen CH, Liu SC, Ho CJ, Shen WW, et al. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol Alcohol. 2008; 43(3): 241-5. [ Links ]
17. Köhler S, Klimke S, Hellweg R, Lang UE. Serum brain-derived neurotrophic factor and nerve growth factor concentrations change after alcohol withdrawal:preliminary data of a case-control comparison. Eur Addict Res. 2013; 19(2): 98-104. [ Links ]
18. Barak S, Wang J, Ahmadiantehrani S, Ben Hamida S, Kells AP, Forsayeth, et al. Glial cell line-derived neurotrophic factor (GDNF) is an endogenous protector in the mesolimbic system against excessive alcohol consumption and relapse. Addict Biol. 2015; 20(4): 629-42. [ Links ]
19. Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009; 33(6): 1012-24. [ Links ]
20. Davies DL, Bortolato M, Finn DA, Ramaker MJ, Barak S, Ron D, et al. Recent advances in the discovery and preclinical testing of novel compounds for the prevention and/or treatment of alcohol use disorders. Alcohol Clin Exp Res. 2013; 37(1): 8-15. [ Links ]
21. Saatcioglu O, Evren C, Cakmak, D. Alcohol use disorders identification test the validity and reliability. Türkiye'de Psikiyatri. 2002; 4(2-3): 107-13 (Turkish). [ Links ]
22. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993; 88(6): 791-804. [ Links ]
23. Evren C, Flannery B, Çelik R, Durkaya M, Dalbudak E. Reliability and validity of Turkish Version the Penn Alcohol Craving Scale (PACS) in male alcohol dependent inpatients. Journal of Dependence. 2008; 9: 128-134 (Turkish). [ Links ]
24. Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999; 23(8): 1289-95. [ Links ]
25. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989; 84(11): 1353-7. [ Links ]
26. Huang MC, Chen CH, Liu HC, Chen CC, Ho CC, Leu SJ. Differential patterns of serum brain-derived neurotrophic factor levels in alcoholic patients with and without delirium tremens during acute withdrawal. Alcohol Clin Exp Res. 2011; 35(1): 126-31. [ Links ]
27. Barker JM, Taylor JR, De Vries TJ, Peters J. Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain Res. 2015; 1628(Pt A): 68-81. [ Links ]
28. Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters.Genomics. 2007; 90(3): 397-406. [ Links ]
29. Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33(5): 849-53. [ Links ]
30. Jung ME, Metzger DB. Alcohol withdrawal and brain injuries: beyond classical mechanisms. Molecules. 2010; 15(7): 4984-5011. [ Links ]
31. Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002; 109(2): 143-8. [ Links ]
32. Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009; 94(10): 1062-9. [ Links ]
33. Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009; 42(6): 270-6. [ Links ]
![]() Correspondence:
Correspondence:
Mehmet Bülent Sönmez
Assistant Professor, MD
Trakya University, School of Medicine
Department of Psychiatry, Edirne
22030, Turkey
Phone: +90 536 445 49 63
Fax: +90 (0284) 235 76 52
E-mail: mbsonmez76@hotmail.com
Received: 8 September 2015
Revised: 29 December 2015
Accepted: 15 January 2016