Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos de la Sociedad Española de Oftalmología
versión impresa ISSN 0365-6691
Arch Soc Esp Oftalmol vol.81 no.12 dic. 2006
EDITORIAL
Intravitreal bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulnes for the treatment of diabetic macular edema
Sistema intravítreo bioerudivel de liberación prolongada de microesferas de triamcinolona (RETAAC). Comunicación preliminar de su utilidad potencial para el tratamiento del edema macular diabético
Cardillo J.A.1, Souza-Filho A.A.2, Oliveira A.G.3
1 MD. Advanced Retinal Diagnose and
Treatment Unit, Hospital de Olhos de Araraquara, Araraquara-SP, Brazil.
Department of Ophthalmlology. Section of Ocular Pharmacology, UNIFESP-EPM, Sao
Paulo-SP, Brazil.
2 PharmD. Department of Ophthalmology, Section of Ocular Pharmacology,
UNIFESP-EPM, Sao Paulo-SP, Brazil.
3 PharmD, PhD. School of Pharmaceutical Sciences, Sao Paulo State
University - UNESP, Araraquara, Sao Paulo-SP, Brazil.
E-mail: hospitaldeolhos@uol.com.br
Macular edema affects approximately 29% of diabetic patients with disease duration over 20 years or more and is responsible for a significant degree of vision loss in this population (1). The Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated a significant benefit of focal laser photocoagulation for the treatment of clinically significant macular edema (2). However, eyes with diffuse macular edema carry a particularly poor prognosis, and laser photocoagulation is at best supportive rather than curative (2,3) prompting interest for alternative therapeutic approaches.
Recently, intravitreal (IVT) administration of triamcinolone acetonide (TA) has provided the ophthalmologist with an extra tool for the treatment of eyes with diffuse diabetic macular edema (4). Despite its apparent established efficacy, concerns such as an unacceptable short duration-effect, and reinjection-related problems (vitreous hemorrhage, endophthalmitis, and retinal detachment) still remain. To theoretically boost and prolong therapeutic effects, the simple solution of injecting higher intraocular triamcinolone doses (usually 25 mg) have been suggested, but at the added expense of enhanced side effects. Clearly, a more rational and optimized medical management strategy utilizing intravitreal triamcinolone targeting diabetic macular edema is still mandatory.
Addressing the relative short half-life of most medications in their free form for intravitreous use, a variety of controlled (sustained)-release drug systems have been studied enthusiastically since the latter half of the 1980s. These systems are capable of delivering drugs over longer time periods than conventional formulations and may add rational to the pharmacologic strategy of treating chronic retinal diseases. Growing evidence is indicating the usefulness of biodegradable microspheres for vitreoretinal drug delivery; offering an excellent alternative to lessen the risk associated with multiple intravitreous injections (5). These erodible devices have the inherent advantage over the non-erodible systems in that they gradually disappear from the site of implantation. In addition, microspheres have also the benefit over larger devices in that they can be delivered by a simple injection, and as a result, fulfilling most of the requirements for an ideal intravitreous-delivery carrier system. On the other hand, the conventional pellet-shape implants require an expensive engineered applicator of invasive delivery nature due to its usually large-gage needles and unfriendly scleral placing requirement. It is also important to ensure that the system chosen for treatment is safe and well-tolerated, eliminating any potential toxic effects. Although, never fully tested in humans, the biodegradable poly (lactic-co-glycolic acid) (PLGA) polymer used in our system has a long history of safety and biocompatibility.
Therefore, our encouraging preliminary experimental results and the lack of clinical studies prompted the authors to investigate the therapeutic response and ocular tolerance of a single intravitreal injection of 1-mg TA in a controlled-release microsphere system (referred in this study as the RETAAC system) in comparison with a single intravitreal injection of 4-mg TA for the treatment of diffuse diabetic macular edema. If it is proved beneficial and safe, this minimally invasive and innovative approach may allow a feasible means for delivering sustained therapeutic quantities of TA to the retina, overcoming the short therapeutic effects the conventional free-form formulation and most of the implantation barriers faced with the conventional pellet system.
Despite limited clinical implication due to a relatively small sample size (9 patients), the present case report is an extension of currently published investigations in that it allows a direct comparison of 1mg-RETAAC versus 4mg TA injection for diffuse diabetic macular edema regarding safety, as well as anatomic and functional outcomes. Overall, a clear long-time trend favoring RETAAC (1 mg) over TA (4 mg) injection was observed. Quantitative measurements of central macular thickness (CMT) by OCT showed a reduction from baseline exceeding 59% (RETAAC) and 56% (TA) at the 1- and 3-month follow-up visits respectively, and the difference in CMT between RETAAC and TA injected eyes was not significant because of a parallel initial pattern of regression of the macular edema. On the contrary, at 6 (P=0.002) and 12 months (P=0.002), RETAAC-injected eyes had thinner CMT measurements compared to TA injected eyes (fig. 1). The beneficial effect of RETAAC on visual acuity was consistent with the reduction on CMT. The gain in visual acuity in the RETAAC- treated eyes averaged 1.6, 2.5, 2.0 and 1.5 lines at 1, 3, 6, and 12 months, respectively. All TA-injected eyes failed to demonstrate a functional outcome response at 6 and 12 months (P=0.04). Therefore, based on the markedly beneficial effect on retinal thickness, as well as a greater functional response, RETAAC administration demonstrated a superior long-term pharmacological performance when compared to TA in this study. In addition, no drug or procedure related side effects were observed in both groups.
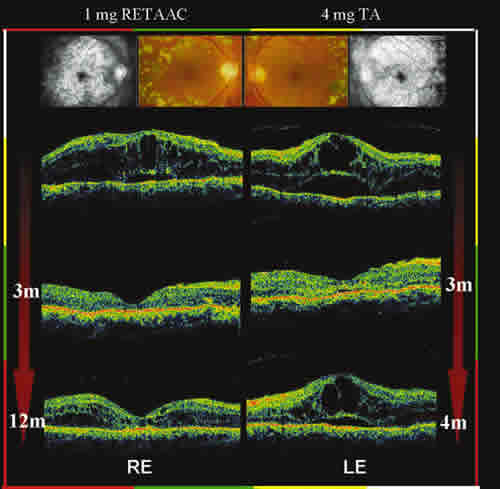
Fig.
1: Diabetic macular edema (ME) thickness evolution of one patient after a
1-mg intravitreal RETAAC injection in the right eye (RE) and a 4-mg
triamcinolone
acetonide (TA) injection in the left eye (LE). Color fundus
photograph after laser
photocoagulation and late-phase angiogram showing cystoid
ME. Baseline six-millimeter
optical coherence tomography (OCT) scans of both
eyes: the central macula has symmetric
thickened appearance, with large
intraretinal hyporeflective cystoid space and serous
retinal detachment. One
month after RETAAC injection (RE), central macular thickness (CMT)
has
dramatically decreased showing a complete resolution of macular edema throughout
the study period (12 months), when a partial CMT relapse is noted. Conversely,
despite an immediate anatomical response, the macular scan of the left eye (TA)
d
isclosed regression of the submacular fluid, cystoid spaces and CMT
measurements
(comparable to baseline) within 4 months following the
intravitreous injection; a CMT
profile that remained practically unaltered
throughout the entire study course.
In conclusion, the findings from this preliminary
report neither advocate nor support the use of triamcinolone-microspheres for
the treatment of diabetic macular edema, but imply that both RETAAC and TA
injections may be well tolerated with long-term performance clearly favoring the
RETAAC (1 mg) over TA (4 mg) for the anatomic and functional aspects of
improvement tested in this groundwork investigation. Targeting chronic diseases,
a rationale for RETAAC as promising approach for the intraocular delivery of
drugs is suggested, and as experience grows guided by the results of appropriate
clinical trials, the precise role of this innovative approach may become more
defined.
References
1. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984; 91: 1464-1474.
2. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985; 103: 1796-1806.
3. Bresnick GH. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology 1983; 90: 1301-1317.
4. Cardillo JA, Melo LA Jr, Costa RA, Skaf M, Belfort R Jr, Souza-Filho AA, et al. Comparison of intravitreal versus posterior sub-Tenon's capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 2005; 112: 1557-1563.
5. Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv Drug Deliv Rev 2001; 52: 5-16.











 texto en
texto en 


