My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO  Similars in Google
Similars in Google
Share
Oncología (Barcelona)
Print version ISSN 0378-4835
Oncología (Barc.) vol.27 n.4 Apr. 2004
CARCINOMA DE ESTÓMAGO
D2 or non D2-lymphadenectomy in gastric cancer
D. Nitti MD; A. Marchet MD; M. Olivieri MD; A. Ambrosi PhD; R. Mencarelli MD; F. Farinati MD; C. Belluco MD; M. Lise MD
Department of Oncological and Surgical Sciences. Clinica Chirurgica 2. University of Padova. Italy
Introduction
The main treatment modality for gastric carcinoma, which is still the second most common cause of cancer-related deaths worldwide, is surgery. In Western countries, a radical surgical procedure can be achieved in 70-80% of cases with a survival at 5 years of 35-40%1-3. However, even after radical procedures, recurrences occur in 50-60% of cases4. Patients with relapse mainly present distant metastasis, but in 20- 40% of cases, recurrences are loco-regional and involve the "gastric bed", the lymph nodes and/or the anastomosis or the duodenal stump4. Loco-regional recurrences are probably related to the biological characteristics of the tumor but, in some cases, they may be due to an inadequate surgical procedure.
The results reported by Japanese surgeons are better than those reported in Western countries for all disease stages, with a 5-year survival rate approaching 50%5. It has been suggested that the use of a different surgical procedure may explain this difference in survival of patients with the same disease stage. In Japan, standard guidelines have been established for lymph node dissection6: extended lymphadenectomy is routinely used, and five-year survival rates after extended lymphadenectomy (D2) reported in retrospective studies are significantly higher than those reported following a lymphadenectomy limited to the perigastric lymph nodes (D1)7.
D2 lymphadenectomy is used less widely in the West. In an overview on gastric cancer treatment in the USA, D2 dissections were performed in only 4 to 7% of cases2, 8-9. The scepticism expressed by Western surgeons may be justified in view of the doubts regarding the therapeutic value of extended lymphadenectomy, strengthened by previous unfavourable results in Western countries9, and by the negative impact the procedure was found to have on survival in previous randomized clinical trials10-11, although conducted on small series. Moreover, extended lymphadenectomy is difficult to perform, requires a longer operating time and incurs a high incidence of mortality and morbidity. One randomized clinical trial in England12 and another in Holland13, confirmed that there is a significant increase in the mortality and morbidity rates after D2 lymphadenectomy with respect to D1 (in the English study, morbidity was 28% for D1 and 46% for D2 and the mortality rate was 6.5% for D1 and 13% for D2; in the Dutch study, mortality and morbidity were 25% and 4% respectively in the D1 group and 43% and 10% respectively in the D2 group). These differences were related to the high percentage of patients who underwent splenectomy and/or distal pancreaectomy. In the English study there was no evidence that D2 resection benefited overall survival: the five-year survival rate in the D1 and D2 arms was 35% and 33%, respectively. Similar negative survival outcomes following D2 lymphadenectomy were reported in the Dutch study: five-year survival rates were 45% for the D1 group and 47% for the D2 group and cumulative risks of relapse at five year were 43% and 37% for D1 and D2, respectively. However, after a median follow-up of 11 years, a significant survival benefit was observed for N2-positive patients of the D2 group: 20% are still alive14.
However, the results of these multicentric studies may be criticized for the following reasons:
a) the standarization of lymph nodes dissection was doubtful15-16: in 51% of the patients who underwent D2 dissection, no lymph nodes were obtained from two or more stations dissected, and in 36% of the D1 dissections the number of nodes removed was smaller than that required;
b) a large number of distal pancreatectomies and splenectomies were performed, but these procedures can be avoided for a adequate D2 lymphadenectomy;
c) with a view to achieving a high rate of accrual, several hospitals participated in the study, and therefore the median number of patients operated on by each surgeon was very small: the morbidity and mortality of surgical procedures are related to the number of procedures performed by each surgeon.
The prognostic value of extended lymphadenectomy was confirmed in the 5th edition of the TNM (UICC 1997), in which the N classification was modified: it is now based on the number of lymph nodes involved (N0, no lymph nodes metastases; N1, 1 to 6 positive lymph nodes; N2, 7 to 15 positive lymph nodes; and N3, more than 15 positive lymph nodes). Moreover, the UICC has stated that the "histological examination of a regional lymphadenectomy specimen will ordinarily include 15 or more lymph nodes". It is therefore necessary to remove at least 15 lymph nodes in order to be able to classify a patient as "N2 or N3".
The EORTC experience
In 1989, the Gastrointestinal Tract Cancer Cooperative Group (GITCCG) of the European Organization for Research and Treatment of Cancer (EORTC) closed a phase III clinical trial on adjuvant chemotherapy with the FAM regimen. In eight European Countries, 314 patients who had undergone curative resection for stage II or stage III gastric carcinoma were randomized to receive the modified FAM regimen, or no further treatment. A retrospective analysis of this study was made to evaluate the effect of prognostic factors on survival and time to recurrence in relation to the characteristics of patients and tumors, and treatment17. For each patient, an evaluation of regional lymphadenectomy was made on the basis of partial or complete D1, D2 or D3 dissection in relation to tumor site. After comparing surgical and pathology data, lymphadenectomy was classified as "adequate" when the extent of lymph node dissection was wider than lymph node metastatic diffusion, "partially adequate" when these coincided and "inadequate" when the dissection did not encompass metastatic diffusion (random lymph node biopsies) or when there was a fault in the surgical technique (e.g., greater omentum not removed).
Since pathology data were incomplete in 5 out of 314 cases, the series evaluated for quality of surgery consisted of 309 patients. Resection was considered "adequate" in 102 patients (33%), "partially adequate" in 131 (42.4%), and "inadequate" in 76 (24.6%). At univariate analysis, statistically significant differences in survival and time to progression emerged for T, N, disease-stage or adequacy of surgery. Survival was significantly better for patients who had "adequate" than in those who had "partially adequate" and "inadequate" surgery (p<0.001) and the differences in survival between "adequate" and "inadequate" or "partially adequate" surgery persisted when stage II and stage III patients were analyzed separately.
Multivariate analysis retained preoperative Hb level, T, N, and adequacy of surgery for duration of survival, and T, N, adequacy of surgery and adjuvant chemotherapy for time to recurrence. These data, show that inadequate lymphadenectomy may result not only in stage shifting, but also in compromised radicality in patients with "curable" disease17.
In the nineties the EORTC evaluated the efficacy of the FAMTX regimen versus surgery alone in a randomized clinical trial on patients with radically resected gastric cancer. At that time, the International Collaborative Cancer Group (ICCG) was also investigating 5-FU+Epirubicin (EPI)+ MTX with Leucovorin rescue (FEMTX) versus surgery alone. The two trials were considered similar enough by an Independent Data Monitoring Committee to be analyzed together. From July 1990 to March 1998, 397 untreated patients were randomised in both trials. Two hundred and six patients from 23 EORTC institutions (trial opened in February'91) and 191 from 16 ICCG institutions were registered. At a preliminary analysis, the overall survivals in the EORTC trial appeared better than those in the ICCG trial18.
An analysis of the surgical procedures used in the two trials showed important differences between extent of surgery. In the EORTC trial 89% of patients underwent D2 dissection. The median number of lymph nodes removed was 13 in the ICCG study, whereas 74% of patients had 13 or more nodes removed in the EORTC trial. Although a direct comparison cannot be made, it appears likely that the more radical surgery performed in the EORTC study resulted in a greater survival benefit than the less radical surgery performed in the ICCG trial18 .
Personal experience
To evaluate the impact of D2 lymphadenectomy on the survival of patients who undergo radical resection for gastric cancer and to demonstrate the role of the other prognostic factors, recently we reviewed the data of 445 consecutive patients operated on at Clinica Chirurgica II, Padova University19-20.
Between February 1980 and December 1999, among 445 patients with histologically confirmed carcinoma of the stomach, 314 patients underwent radical resection (R0). Thirty-seven were excluded from the analysis: the study is therefore based on data from 277 patients who underwent radical resection (R0). D2 dissection was performed in 259 (93.6%), and D1 lymphadenectomy in 18 (6.4%) cases.
From 277 patients, a total of 7,668 lymph nodes (median 27; mean, 27.6; range 11-62) were removed and examined. Lymphadenectomy included more than 15 lymph nodes was performed in 245 cases (88.4%): 11 to 25 nodes in 116 patients (41.8%) and more than 25 nodes in 161 patients (58.2%). A total of 1,280 lymph nodes were metastatic (median, 5; mean, 8.25; range, 1-47). For the 277 patients, the 5-year survival rate was 57%; it was 82% for the 117 patients without lymph node metastases, and 37% for the 160 patients with lymph node metastases (Fig. 1). Table I reports findings at univariate analysis for prognostic factors.
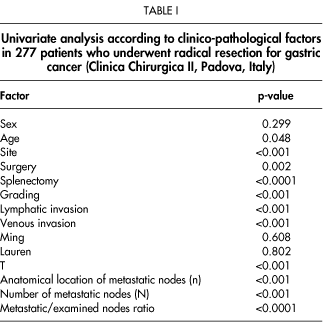
When multivariate analysis was performed, only age, T, and metastatic/examined lymphnodes ratio (N ratio) were found to be independent prognostic factors (RR of N ratio 1=1.60, RR of N ratio 2=1.72; RR of N ratio 3=5.52) (Table II).
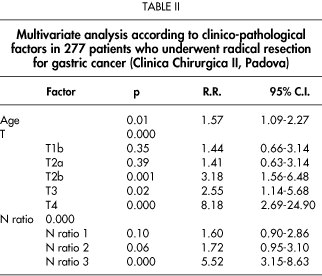
In our study, which considered almost exclusively D2 lymphadenectomy, the overall survival rate appears better than that usually reported in western countries, and similar to that reported in Japanese trials. These results could be related to the quality of surgery performed in our series. We therefore believe that D2 lymphadenectomy allows accurate disease staging of the disease and can be "curative" in patients with gastric cancer confined to the locoregional area.
References
1. Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of US gastric carcinoma patients treated with gastrectomy. Fifth Edition American Joint Committee on Cancer Staging, proximal disease, and the "different disease" hypothesis. Cancer 2000; 88:921-32. [ Links ]
2. Berrino F, Sant M, Verdecchia A, et al. Survival of cancer patients in Europe. The Eurocare study. IARC Sci, 1995; Publ. No.132. [ Links ]
3. Siewert JR, Bottcher K, Roder JC, Busch R, Hermanek P, Meyer HJ. Prognostic relevance of lymph node dissection in gastric carcinoma. German Gastric Carcinoma Study Group. Br J Surg 1993; 80:1015-8. [ Links ]
4. Gunderson LL. Gastric cancer. Patterns of relapse after surgical resection. Seminars in Radiation Oncology 2002; 12:150-61. [ Links ]
5. Harrison LE, Karpeh MS, Brennan F. Extended lymphadenectomy is associated with a survival benefit for node-negative gastric cancer. J Gastrointest Surg 1998; 2:126-31. [ Links ]
6. Noguchi Y, Imada T, Matsumoto A, Coit DG, Brennan MF. Radical surgery for gastric cancer: a review of the Japanese experience. Cancer 1989; 64:2053-62. [ Links ]
7. Maruyama K, Gunven P, Okabayashi K, Sasako M, Kinoshita T. Lymph nodes metastases in gastric cancer. Ann Surg 1989; 210:596-602. [ Links ]
8. Hundal SA, Macdonald JS, Benedetti J, Fitzsimmons T, for the Southwest Oncology Group and the Gastric Intergroup. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol 2002; 9:278-86. [ Links ]
9. Wanebo HJ, Kennedy BJ, Winchester DP, Fregmen A, Stewart AK. Gastric carcinoma: does lymph node dissection alter the survival? J Am Coll Surg 1996; 183:616-24. [ Links ]
10. Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 1988; 75:110-2. [ Links ]
11. Diggory RT, Cuschieri A. R2/R3 gastrectomy for carcinoma: an audited experience of a consecutive series. Br J Surg 1985; 72:146-8. [ Links ]
12. Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer. Long term results of the MRC randomised surgical trial. Br J Cancer 1999; 79:1522-30. [ Links ]
13. Bonekamp JJ, Hermans J, Sasako M, van de Velde CJH. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340:908-14. [ Links ]
14. Peeters K., van de Velde CHJ. Improving treatment outcome for gastric cancer: the role of surgery and adjuvant therapy. J Clin Oncol 2003; 21(23s):272s-3s. [ Links ]
15. Brennan MF. Lymph node dissection for gastric cancer. New Engl J Med 1999; 340:956-8. [ Links ]
16. Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg1997; 84:1567-71. [ Links ]
17. Lise M, Nitti D, Marchet A, Sahmoud T, Duez N, et al. Prognostic factors in resectable gastric cancer: results of EORTC study N°40813 on FAM adjuvant chemotherapy. Ann Surg Oncol 1995; 2:495-501. [ Links ]
18. J. Wils, D. Nitti, J. Guimaraes-Dos-Santos, G. Fountzilas, F. Conte, C. Sava, A. Tres, E. Sanchez, J. Homewood, M.L. Couvreur, E. Hall, B. Baron, M. Lise. Randomized phase III studies of adjuvant chemotherapy with FAMTX or FEMTX in resected gastric cancer. Pooled results of studies from the EORTC GI-group and the ICCG. Proceedings of ASCO 2002. Abstract 521. [ Links ]
19. Nitti D, Marchet A, Belluco C, Olivieri M, Ambrosi A, Mammano E, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol 2003; 10:1077-85. [ Links ]
20. Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Farinati F, Belluco C, Lise M. Limphadenectomy in patients with gastric cancer: a critical review. Tumori 2003; 2:S35-S38. [ Links ]
 Correspondence to
Correspondence to
Prof. Donato Nitti
Istituto di Clinica Chirurgica Generale II
Università di Padova
Via Giustiniani, 2
35128 - Padova, Italy
E-mail: donato.nitti@unipd.it














