Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO  Similares em Google
Similares em Google
Compartilhar
Oncología (Barcelona)
versão impressa ISSN 0378-4835
Oncología (Barc.) vol.27 no.6 Jun. 2004
CONTROL DE CALIDAD
Utilisation of portal imaging in positional control
V. Khoo
Royal Marsden Hospital St. George's Hospital & Christie Hospital United Kingdom
SUMMARY
Treatment verification is crucial for precision radiotherapy irrespective of the treatment techniques being used. Current methods of electronic portal imaging has provided quality assured standards in modern radiotherapy. However there are now significant advances in on-line 3D imaging such as kv imagers and cone beam CT acquisition. There is an opportunity to obtain 3-D images with soft-tissue definition at all anatomical sites treated with external beam radiation therapy at acceptably low radiation doses. Image quality will be dependent on tumour site and dose delivered. There is much on-going work in optimizing image capture using this new technology. This technology has the potential for optimising generic population margins, customising and adapting treatments for individual patients, and accurate verification of complex treatment delivery. This technology may revolutionise current quality assurance programs and permit the development of new protocols for adaptive, predictive, gated or tracking in radiotherapy.
Introduction
Radiotherapy treatment is a sophisticated chain of technical events that relies substantially on the ability to locate the target volume within the patient with precision. Some of the major steps in the radiotherapy treatment chain are outlined below:
-
Patient immobilisation.
-
Treatment planning.
-
Determination of tumour and normal tissues volumes.
-
Simulation.
-
Radiotherapy delivery.
-
Treatment verification.
Accurate, reliable and appropriate images are needed to ensure that staging of disease extent, delineation of volumes of interest such as tumour bearing regions and identification of organs-at-risk (OAR), organisation of treatment planning, and verification for radiotherapy are optimal. This is especially important in three dimensional (3D) radiotherapy where conformal (CFRT) or intensity modulated (IMRT) techniques are being used. However there are numerous uncertainties that can be present within this chain of events of the patient's treatment pathway from the determination of treatment volumes to patient setup and during treatment delivery for a radical course of daily fractionated radiotherapy.
Radiotherapy Treatment and Delivery Associated Errors
The important sources of errors may categorised and grouped in the following the list and will be discussed later on.
-
Patient group, equipment or protocol related errors
-
Treatment preparation errors (systematic)
-
Delivery related errors (random)
-
Intra-fractional errors (physiological activity)
When we look more closely at the geometrical steps where uncertainties may compound positional errors in the design and delivery of radiotherapy, this can be illustrated by the following exemplars.
When the patient is computed tomography (CT) scanned for planning in the treatment position, skin tattoos are marked on the patient's skin and aligned with the CT room lasers. These lasers are then aligned with the CT room coordinates. This then represents the treatment set-up upon which subsequent radiotherapy is determined. However during radiotherapy delivery itself, the patient's set-up from the CT room needs correlation with the treatment room lasers. There may also be 3D positional changes between the skin marks and patient's bony anatomy as well as the position of the tumour relative to the patient's bony anatomy.
It is also important to measure and determine the magnitude of treatment errors, both systematic and random. One common and current method to determine the magnitude of treatment positional errors is portal imaging. This will also enable the reduction of positional errors. Portal imaging has been compared to simulation images but ideally should be correlated to digital reconstructed radiographs to enable verification of beam position and bony anatomy. It will be clear to any clinical practitioner that the pattern of errors will differ depending upon the type of tumour being treated and this is directly related to the location of the anatomical organ or region.
Planning Margins
Treatment margins are an important aspect of treatment planning. Determination of the margin needed is a careful consideration. Inadequate margins will result in lower local control of the tumour and impact on survival whereas too large a margin will result in excessive irradiation of normal tissues or organs leading to unacceptable rates of treatment related complications. There are various algorithms currently available for the determination of target and normal organ treatment margins that account for the influence of both systematic and random deviations. This will enable more reliable provision of treatment margins from the clinical target volumes (CTV) to planning target volumes (PTV).
There are many conceptual methods of margin provision and thus it is clear that there is a lack of consensus on the optimum method. Simulations of real patient data have been reported to ascertain the impact of geometrical variations on dosimetry but these assessments are very tumour and site specific (Killoran et al 1997, Mageras et al 1996). One method designed to be generalisable utilises computation of the probability distribution and involves the analysis of each possible treatment preparation error to the dose distribution on the CTV generating dose population histograms (van Herk et al 2002). The resulting simplistic PTV margin recipe to cover the CTV for 90% of the patients with the 95% isodose can be outlined as follows:
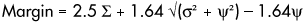
Where approximated y » 3.2 mm
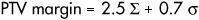
å = standard deviation of all systematic errors combined quadratically
s = standard deviation of all random errors combined quadratically
y = standard deviation (width) of penumbra (Gaussian)
The margin recipe for normal organs (McKenzie et al 2002) can be outlined as follows:

Rationale for image guidance
Another important source of error is organ motion. New advances with on-line imaging can now provide improved image guidance. Advanced planning techniques such as IMRT permit the creation of highly conformal dose distributions. These planning techniques are based on a planning 'snap shot' of the tumour position at the time of the planning CT scan. The derived PTV based on this planning CT scan may or may not be representative of the average location of the tumour and certainly cannot account for the exact location of the target at the time of radiation. Currently our capacity to design such intricate and precise treatment plans exceeds our ability to reliably and accurately deliver them. Therefore the benefit of conformal and intensity-modulated radiotherapy techniques is still limited by uncertainties in both patient set-up and internal organ motion.
Even if treatment set-up is reliable, the patient is cooperative and he/she can be comfortably stabilised for a period of time, organ motion will occur due to respiration and internal physiological movement such as respiration, involuntary muscle contracture, filling and emptying of organs (stomach, bowel, bladder and rectum). These physiological movements can alter the relative 3D spatial position of the PTV and the adjacent OAR compared to the initial CT planning scan on subsequent days of a curative course of fractionated radiotherapy. This movement, if not accounted for in the treatment volume, may result in geographical miss with reduced local control of the tumour. Treatment margins are usually prescribed to avoid such geographical miss but large planning margins for target and localisation uncertainty negate the value of CFRT/IMRT techniques and increase the potential for late tissue damage particularly if the OAR are irradiated to a higher dose than anticipated by being displaced into the high dose radiation region. This has implications for radiotherapy regimes of dose escalation.
It is clear that the uncertainty in daily tumour localisation precludes accurate delivery of radiation during a course of radical fractionated treatment delivered over many days. This is particularly pertinent in anatomical sites where internal organ motion is present and of substantial magnitude. These motion effects urgently need to be understood and handled realistically, particularly at soft tissue sites, before they undermine the further benefits expected from CFRT and IMRT, dose escalated regimes or more sophisticated strategies that are designed for biological boosting of disease.
Adequate definition of the 3D spatial position of the intended tumour volume is needed not only online on a daily basis but in real-time during radiotherapy in order to provide the accuracy and reliability that is currently lacking in modern radiotherapy. Online evaluation of the target position and 3D knowledge of the target motion may also permit appropriate reductions in the planning margins that are currently used in many anatomical sites with the benefit of reducing normal tissue morbidity. Herein lies the rationale for image guidance in radiotherapy.
Image Guided Strategies
The implications of internal organ motion are site specific and depend on the anatomical region in question for example treating breast and lung cancers will require knowledge of the respiratory cycle in the delivery of radiotherapy whereas radiation of the prostate will require knowledge of the surrounding pelvic organs and their physiological activity such as rectal and bladder filling and emptying. It is important to define the magnitude, distribution, pattern, and type of organ motion for each anatomical region and its impact on the tumour situated within that region. In some situations, organ deformation is also an important issue. This is particularly relevant for organs that fill or empty such as the lungs, stomach, bladder and rectum. In order for image guidance to be fully effective, 3D anatomical information is needed to enable adequate implementation of any image guided program. This task has now been made easier with the recent developments in treatment imaging.
One of the new innovations in on-line imaging include kilovoltage (kV) cone-beam imagers mounted on the LINAC gantry permitting kV radiography that may provide improved superior bony identification compared to EPI megavoltage identification (Jaffray et al 2002). Importantly, it can also provide cross-sectional imaging for online 3D verification and online fluoroscopy for real-time assessment of target and organ motion for dynamic tracking and gating similar to robotic LINACs. Online 3D target motion knowledge may allow treatment margin reductions with benefits of reducing normal tissue morbidity. Cone beam imaging may also be correlated with functional imaging for response evaluations for selective management. Some examples using this technology will be discussed later on.
Radiotherapy in the thorax
This is a site that is plagued by known physiological motion that is significant in its magnitude. Tumours located in the thorax such as lung and breast cancers can be displaced by both respiratory and cardiac motion. Respiratory motion can be monitored or made predictive in order to development methods to sensibly deal with this motion. There are several techniques available to deal with reducing the effect of respiratory motion such as central abdominal compression, active breathing control (ABC) device or the use of external thorax infra-red marker systems for monitoring respiratory cycles.
The use of the active breathing control (ABC) device allows for voluntary control of the respiratory cycle by the patient. Using the ABC device, gating treatment methods can be introduced by asking the patient to breath hold for the radiotherapy treatment in lung and breast cancers as well as Hodgkin's disease (Wong et al 1999, Stromberg et al 2000). The surface of the thorax may also allow the use of surface guided active monitoring of the respiratory cycle or the use of externally placed infra-red marker systems that can measure the breathing cycle and amplitude of oscillation. Recent studies suggest that whilst values may vary substantially between different patients, there is consistency and stability for the individual patient within each daily treatment or during a course of treatment. These techniques can lead to programs of free-breathing phase gated radiotherapy (Korreman et al 2003).
Bladder Radiotherapy
The position of pelvic tumours can be affected by movement of the bowel, filling and emptying of the bladder and rectum. Bladder cancer is a good example. Significant variation in physiological activity with bladder filling/emptying as well as bladder motion has been reported (Turner et al 1997, Meijer et al 2003). In a study of 30 patients imaged sequentially during a curative course of radiotherapy, a change in bladder area of up to 55% was recorded despite a consistent protocol of bladder emptying prior to scanning (Turner et al 1997). In addition, 60% of cases demonstrated bladder wall movement of greater than 15 mm relative to the 95% prescribed isodose line. This data illustrates that significant movement can occur during radiotherapy and simply increasing the treatment margin to encompass this variation is not an optimum solution.
There are adaptive and predictive methods to enable 3D changes in target volume to be tailored to the individual patient. Adaptive radiotherapy methodology utilises initial assessment of daily bladder treatment volumes to select a smaller range of treatment plans most likely to represent the majority of daily shapes in each patient. Using online daily assessment the best fit bladder plan is selected from an acceptable range of bladder plans for use each day. Predictive radiotherapy methodology necessitates the evaluation of the individual patient's pattern of volume enlargement and deformity. This can be performed using cine magnetic resonance imaging. The extent of change depends on several factors that are both patient and tumour dependent. Using time dependent patterns of individual patient's bladder deformity, treatment volumes may be predicted on a daily basis. Both these methods are not mutually exclusive and each method has its respective pros and cons. However, in order to implement either technology, 3D methods of on-line assessment are needed to provide the necessary 3D data.
Prostate Radiotherapy
Radiotherapy is an important form of curative treatment for both early and locally advanced prostate cancer. Prostate radiotherapy is another good example where prostate organ motion and patient set-up error are important uncertainties that need to be addressed in the external beam irradiation of the prostate gland during a fractionated course of radiotherapy. This is particularly pertinent as many dose escalated programs using IMRT have been utilised in this disease.
It is well known that the anatomical position of the prostate gland and seminal vesicles can be affected by the physiological movements of the surrounding pelvic organs such as the rectum and bladder. Studies have assessed prostate movement using repeated computed tomography (CT) scans over the course of radiotherapy (Ten Haken et al 1991, van Herk at al 1995, Beard et al 1998, Dawson et al 1998, Zelefsky et al 1999). Some investigators have used radiographic films with or with CT and aided by implanted radio-opaque prostatic markers (Balter et al 1995, Crook et al 1995, Vigneault et al 1997). From these reports, the mean inter-fraction displacement of the prostate gland has been reported to range between 3 to 7 mm with the majority of movement in the antero-posterior axis followed by the superior-inferior axis. More recently, potential intra-fraction movement in prostate cancer patients has been assessed using cine MR (Padhani et al 1999, Huang et al 2002, Mah et al 2002, Nederveen et al 2002). The median prostate displacement was reported to be in the anterior direction of 4.2 mm and in 16% of patients, the movement was greater than 5 mm (Padhani et al 1999). More recent studies have reported that the average intra-fraction displacement is smaller than 4 mm. For the individual patient, prostate positional changes may be substantially larger then the reported average displacement for the population under study. These changes in tumour position for the individual need to be accounted for with larger treatment margins which can be avoided if real-time tracking and gating of the treatment beam is available.
Various monitoring strategies both off-line and online with adaptive or real-time manipulation of the treatment delivery have been utilised. Immobilisation of the rectum using rectal balloon have been used to more reliably reduce the interaction of the rectal displacement of the prostate (Wachter 2001). Daily relocalisation of the target prior to treatment has been used using a stereo-coordinated ultrasound device linked to the treatment bed (Lattanzi et al 1999, Lattanzi et al 2000, Chandra et al 2003). There is some early work performed to use implanted markers for tracking in prostate cancer in Japan (Shimizu et al 2000, Kitamura 2002). This system requires specialized installation of stereotactically orientated image intensifiers housed within the floor and ceiling of the treatment delivery room to spatially coordinate the location of implanted markers. One potential advantage of using a cone beam system such as that from the Elekta Synergy or Varian On-Board Imager is that it will not required such specialised bunker room installation or increased room space as it is mounted on the linear accelerator. In this manner, this technology can be more easily transported to radiotherapy departments world-wide.
Dose escalation hypofractionated regimes using IMRT are being studied for prostate cancer patients at both the Christie and Royal Marsden Hospitals in anticipation of improved cure rates and reduced morbidity by exploiting the low (/( ratio reported in prostate cancer. Reliable accurate targeting of the prostate gland is thus necessary to improved localisation and reduce radiation dose to the adjacent rectum and bladder. The ability to track the prostate PTV in real time during treatment delivery and provide beam gating will be an important development to ensure that the potential of dose escalation with CFRT or IMRT techniques is realised. This technique will also address the essential question of treatment verification and allow quality assurance of the treatment program.
Reference
Balter JM, Sandler HM, Lam K, Bree RL, Lichter AS, Ten-Haken RK (1995). Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys 31:113-8. [ Links ]
Beard CJ, Kijewski P, Bussiere M, Gelman R, Gladstone D, Shaffer K, et al (1996). Analysis of prostate and seminal vesicle motion: implications for treatment planning. Int J Radiat Oncol Biol Phys 34:451-8. [ Links ]
Chandra A, Dong L, Huang E, Kuban DA, O'Neill L, Rosen I, Pollack A (2003). Experience of ultrasound-based daily prostate localization. Int J Radiat Oncol Biol Phys. 1;56(2):436-47. [ Links ]
Crook JM, Raymond Y, Salhani D, Yang H, Esche B (1995). Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother Oncol 37:35-42. [ Links ]
Dawson LA, Mah K, Franssen E, Morton G (1998). Target postion variability throughout prostate radiotherapy. Int J Radiat Oncol Biol Phys 42:1155-61. [ Links ]
Huang E, Dong L, Chandra A, Kuban DA, Rosen II, Evans A, Pollack A (2002). Intrafraction prostate motion during IMRT for prostate cancer. Int J Radiat Oncol Biol Phys 53(2):261-8. [ Links ]
Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA (2002). Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 1;53(5):1337-49. [ Links ]
Killoran JH, Kooy HM, Gladstone DJ, Welte FJ, Beard CJ (1997). A numerical simulation of organ motion and daily setup uncertainties: implications for radiation therapy. Int J Radiat Oncol Biol Phys 37:213-21. [ Links ]
Kitamura K, Shirato H, Seppenwoolde Y, Onimaru R, Oda M, Fujita K, Shimizu S, Shinohara N, Harabayashi T, Miyasaka K (2002). Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int J Radiat Oncol Biol Phys 53(5):1117-23. [ Links ]
Korreman SS, Pedersen AN, Specht L, Nystrom H (2003). Breathing adapted radiotherapy (BART) for breast cancer. Radiother Oncol. 68(S1):40. [ Links ]
Lattanzi J, McNeeley S, Hanlon A, Schultheiss TE, Hanks GE (2000). Ultrasound-based stereotactic guidance of precision conformal external beam radiation therapy in clinically localized prostate cancer. Urology. 55(1):73-8. [ Links ]
Lattanzi J, McNeeley S, Pinover W, Horwitz E, Das I, Schultheiss TE, Hanks GE (1999). A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int J Radiat Oncol Biol Phys. 43(4):719-25. [ Links ]
Mageras GS, Kutcher GJ, Leibel SA Zelefsky MJ, Melian E, Mohan R, Fuks Z (1996). A method of incorporating organ motion uncertainties into three-dimensional conformal treatment plans. Int J Radiat Oncol Biol Phys 35:437-45. [ Links ]
Mah D, Freedman G, Milestone B, Hanlon A, Palacio E, Richardson T, Movsas B, Mitra R, Horwitz E, Hanks GE (2002). Measurement of intrafractional prostate motion using magnetic resonance imaging. Int J Radiat Oncol Biol Phys 54(2):568-75. [ Links ]
Martinez AA (1999). The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 44(4):911-9. [ Links ]
McKenzie A, van Herk M, Mijnheer B (2002). Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother Oncol. 62(3):299-307. [ Links ]
Meijer GJ, Rasch C, Remeijer P, Lebesque JV (2003). Three-dimensional analysis of delineation errors, setup errors, and organ motion during radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys 55(5):1277-87. [ Links ]
Nederveen AJ, van der Heide UA, Dehnad H, van Moorselaar RJ, Hofman P, Lagendijk JJ (2002).Measurements and clinical consequences of prostate motion during a radiotherapy fraction. Int J Radiat Oncol Biol Phys. 2002 May 1;53(1):206-14. [ Links ]
Padhani AR, Khoo VS, Suckling J, Husband JE, Leach MO, Dearnaley DP (1999). Evaluating the effect of rectal distension and movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys 44:525-33. [ Links ]
R (2002). The influence of a rectal balloon tube as internal immobilization device on variations of volumes and dose-volume histograms during treatment course of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 52(1):91-100. [ Links ]
Shimizu S, Shirato H, Kitamura K, Shinohara N, Harabayashi T, Tsukamoto T, Koyanagi T, Miyasaka K. Use of an implanted marker and real-time tracking of the marker for the positioning of prostate and bladder cancers. Int J Radiat Oncol Biol Phys 2000; 48:1591-1597. [ Links ]
Stromberg JS, Sharpe MB, Kim LH, Kini VR, Jaffray DA, Martinez AA, Wong JW (2000). Active breathing control (ABC) for Hodgkin's disease: reduction in normal tissue irradiation with deep inspiration and implications for treatment. Int J Radiat Oncol Biol Phys. 48(3):797-806. [ Links ]
Ten-Haken RK, Forman JD, Heimburger DK, Gerhardsson A, McShan DL, Perez-Tamayo C, et al (1991). Treatment planning issues related to prostate movement in response to differential filling of the rectum and bladder. Int J Radiat Oncol Biol Phys 20:1317-24. [ Links ]
Turner SL, Swindell R, Bowl N, Marrs J, Brookes B, Read G, et al (1997). Bladder movement during radiation therapy for bladder cancer: implications for treatment planning. Int J Radiat Oncol Biol Phys 39:355-60. [ Links ]
van Herk M, Remeijer P, Lebesque JV (2002). Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol Biol Phys. 52(5):1407-22. [ Links ]
van-Herk M, Bruce A, Kroes A, Shouman T, Touw A, Lebesque JV (1995). Quantification of organ motion during conformal radiotherapy of the prostate by three-dimensional image registration. Int J Radiat Oncol Biol Phys 33:1311-20. [ Links ]
Vigneault E, Pouliot J, Laverdiere J, Roy J, Dorion M (1997). Electronic portal imaging device detection of radio-opaque markers for the evaluation of prostate position during megavoltage irradiation: a clinical study. Int J Radiat Oncol Biol Phys 37:205-12. [ Links ]
Wachter S, Gerstner N, Dorner D, Goldner G, Colotto A, Wambersie A, Potter R (2002). The influence of a rectal balloon tube as internal immobilization device on variations of volumes and dose-volume histograms during treatment course of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 52(1):91-100. [ Links ]
Wong JW, Sharpe MB, Jaffray DA, Kini VR, Robertson JM, Stromberg JS, Martinez AA (1999). The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 44(4):911-9. [ Links ]
Zelefsky MJ, Crean D, Mageras GS, Lyass O, Happersett L, Ling CC, Leibel SA, Fuks Z, Bull S, Kooy HM, van Herk M, Kutcher GJ (1999). Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol 1999;50:225-34. [ Links ]














