My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO  Similars in Google
Similars in Google
Share
Oncología (Barcelona)
Print version ISSN 0378-4835
Oncología (Barc.) vol.28 n.4 Apr. 2005
ORIGINALS
Quality of life assessment in Spanish lung cancer patients by the EORTC questionnaires
J. I. Arraras Urdaniz; M. Martínez Aguillo; A. Manterola Burgaleta; E. Salgado Pascual; E. Martínez López; R. Vera García; J. J. Illarramendi Mañas
Servicios de Radioterapia y de Oncología Médica. Hospital de Navarra
SUMMARY
PURPOSE: To assess the quality of life of a sample of lung cancer patients during their treatment.
MATERIAL AND METHODS: 170 patients with limited and advanced small-cell lung cancer (SCLC), and with stages III and IV non-small cell lung cancer (NSCLC) were considered. Patients filled the EORTC QLQ-C30 questionnaire, and the EORTC-LC13 lung module at three different times during the treatment and follow-up. Demographic and clinical data were also recorded. We compared the groups according to the stage and treatment modality, and studied the changes of the quality of life throughout time.
RESULTS: The quality of life scores of the patients under the given protocols: were acceptable in the three considered measurements, as were also the toxicity scores. At the base line there are limitations in the areas of symptoms of disease and quality of life. Few differences were observed between the groups at different stages and under the different treatment modalities. The quality of life scores were similar according to the three measurements. Treatment modality showed a specific effect in the second measurement, while in the third one it improved in some toxicity areas and persisted in others.
CONCLUSIONS: The quality of life of the patients receiving the complete protocols was acceptable. The patients under treatment stood it adequately.
Key words: Lung cancer. Chemotherapy. Radiotherapy. Quality of life valuation.
RESUMEN
PROPRÓSITO: el objetivo de este trabajo es evaluar la calidad de vida de una muestra de pacientes con cáncer de pulmón durante el tratamiento.
METODOS: 170 pacientes con cáncer de pulmón, microcítico con enfermedad limitada y avanzada, y no microcítico estadíos III y IV. Los pacientes han contestado el cuestionario EORTC QLQ-C30 y el módulo de pulmón EORTC-LC13 en tres momentos a lo largo del tratamiento y seguimiento. Se han recogido datos demográficos y clínicos. Se han comparado grupos formados en función del estadio y tratamiento, y se han estudiado los cambios en la calidad de vida a lo largo del tiempo.
RESULTADOS: las puntuaciones de calidad de vida de los pacientes que han recibido los protocolos propuestos son aceptables en las tres mediciones al igual que las puntuaciones de toxicidad. En la medición de línea base hay limitaciones en las áreas de síntomas de la enfermedad y la calidad de vida global. Hay pocas diferencias entre grupos organizados según tipos de tratamiento y estadio de la enfermedad. Las puntuaciones de calidad de vida son muy similares en las tres mediciones. Los tratamientos tienen un efecto específico en la segunda medición, que mejora en la tercera en algunas áreas de toxicidad, y en otras persiste.
CONCLUSIONES: la calidad de vida de los pacientes que han recibido los protocolos completos es adecuada. Los pacientes que reciben los tratamientos pueden tolerarlos adecuadamente.
Palabras clave: Calidad de vida. Cáncer de pulmón. Quimioterapia. Radioterapia. Evaluación.
Introduction
Lung cancer is one of the main causes of death among females and males, and its treatment has shown limited progress in recent decades. Quality of Life studies in this area are very important, especially where few medical differences can be expected in the effectiveness of the different treatments. There is a debate around the treatment of lung cancer as to which treatment modality should be administered and until what point in the evolution of the disease. This controversy is more intense in advanced disease. There are cultural issues within this debate as happens with other related variables like the level of the information that is disclosed to patients or the support from relatives. Quality of Life assessment has a major contribution to make to this debate and has the same level of importance as other clinical variables like toxicity or disease progression1. Several studies with lung cancer in its different histologies and spread include an assessment of Quality of Life2-11.
Quality of Life assessment in cancer patients has received increasing interest in recent years. Previously it was primarily traditional biological criteria -tumour response, time to progression, disease free and overall survival- that were the focus of clinical trials12. The European Organization for Research and Treatment of Cancer (EORTC) has a study group on Quality of Life. One of the tasks that this study group has addressed has been the development of questionnaires that assess Quality of Life in international clinical trials. In this sense, the Quality of Life study group decided to create a combined assessment system that included a core questionnaire, which could evaluate issues common to different cancer sites and treatments, and various modules complementing the core questionnaire13-15. These modules include specific aspects of treatments or disease sites: breast, head and neck and others16-19. A second generation of the core questionnaire, the QLQ-C30, has been used in multiple psychometric and clinical studies20-23. Besides, the EORTC Quality of Life group has recently developed the third version of this instrument19. The first module that was created was the lung cancer module, QLQ-LC1324. This module was developed to assess the specific symptoms of lung cancer and its treatment that were not covered at all, or insufficiently, in the core questionnaire. These EORTC questionnaires (core and lung modules) are considered adequate to assess the Quality of Life (QoL) of lung cancer patients25-26.
The Oncology Departments of the Hospital of Navarre have long experience in Quality of Life research. Members of these departments have been continuously participating in different projects of the EORTC Quality of Life study group since 1992. Besides, clinical studies with different tumours, in which Quality of Life has been one of the main outcome variables, have been carried out in both departments27.
The aims of the present study are to assess the biographic and clinical data of a sample of lung cancer patients with un-resectable disease, to study their QoL at different points in the treatment process, to assess changes at these treatment points and to evaluate differences between groups based on stage of disease and treatment modalities.
We expected the quality of life of the patients who fill in the questionnaires in the different assessment points to be moderately high, and to find few differences between groups based on stage of the disease or treatment modality. We also expected some reductions in QoL during the treatment process that could improve in the follow-up period.
Materials and methods
Patients
An initial sample of 186 consecutive un-resectable lung cancer patients was recruited. These patients started their treatment at the Oncology Department of the Hospital of Navarre between January 1997 and June 1998. This sample of patients was subdivided into 4 groups: a) small cell lung cancer patients with limited disease and b) non-small cell stage III lung cancer patients, who were receiving a treatment of chemo-/radiotherapy; c) small cell lung cancer patients with advanced disease and d) non-small cell stage IV lung cancer patients, who were undergoing chemotherapy. Patients with a life expectancy lower than three months or with cognitive limitations were excluded. Cognitive function was assessed by a psychologist using selected questions from the Mini-Mental State Examination28.
Evaluation
Patients completed the EORTC core questionnaire QLQ-C30 (version 2.0) and the lung cancer module QLQ-LC13 (Table I). Both instruments have been validated for use in Spain29-30. In both instruments the scores range between 0 and 100. In the core, higher scores represent better levels in the functioning areas and the global scale, and higher disturbance on the symptom scales/items. In the module, higher scores represent higher levels of symptomatology. The various scales and items of these instruments are considered as the main endpoints of this study.
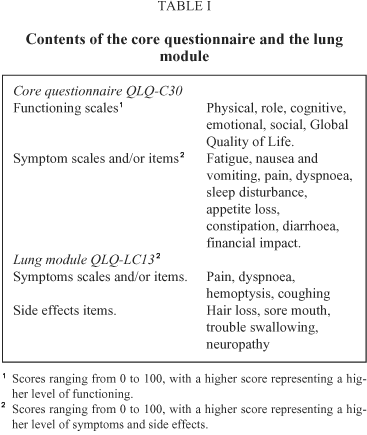
After obtaining verbal consent, patients filled in these questionnaires at three different points: 1) at base-line on the first day of treatment; 2) the last day of the second chemotherapy cycle to study the effects of part of the treatment; 3) one month after the end of the treatment, at a follow-up visit, to study the cumulative effect of the treatment and the disease state. We have established these assessment points in order to provide a view of Quality of Life during the whole treatment process and after considering factors such as patient fatigue and their interest in answering these instruments. Each patient tried to complete the questionnaires themselves. If this was not possible but could be done with assistance, or if they preferred so, they were helped by a psychologist. Those cases in which the patient did not fill in the questionnaire were considered as missing data, and the reasons were recorded. Those questionnaires in which 30% of the items were not answered were excluded. We have also recorded patients' age, gender, and clinical data: initial weight loss (none, lower and higher than 10%), spread and histology of the disease at the first assessment. Biographic and clinical data were taken from the clinical record. Performance status was assessed by the physician at the three time points with the Karnofsky scale31, as well as level of toxicity with selected items from the Common Toxicity Criteria32 at the second and third measurement. We have recorded the treatment modality proposed for patients who have completed just the first assessment and the actual treatment received in the rest of the sample (radiotherapy, chemotherapy or combined).
Treatment schedule
The protocol treatment for the small cell lung cancer with limited disease group consisted of hyperfractionated radiotherapy 150cGy per session twice daily to a total dose of 45 Gy. Concurrent chemotherapy was initiated on day one of radiotherapy with etoposide 80mg/m2 a day, days 1-3-5 and cisplatin 20 mg/m2 a day, days 1 to 5. This cycle was repeated every 21 days until completing a total of four cycles. The protocol treatment for the small cell lung cancer group with advanced disease consisted of chemotherapy of etoposide 80 mg/m2 a day X 3 days and cisplatin 80 mg/m2 X 1 day. Cycles were administered each 21 days up to a total number of 4 to 6 depending on treatment tolerance and response.
The non-small cell stage III lung cancer group received a neoadjuvant chemotherapy cycle of the MVP scheme: cisplatin 120 mg/m2 on day 1, mitomicine C 10 mg/m2 on day 1, vindesine 3 mg/m2 on days 1, 8, 15 and 22. Radiotherapy was initiated between days 30 and 40 with hyperfractionated treatment of 120 cGy twice daily to a total dose of 6960 cGy. A concomitance was performed on days 1 and 5 of radiotherapy with cisplatin 20 mg/m2 a day by continuos infusion. This was repeated in the last week of radiotherapy, if the patient's general condition allowed it33. The protocol for the non-small cell stage IV group consisted of cisplatin 80-100 mg/m2 on day 1 and vindesine 3 mg/m2 on days 1, 8 and 15. Four chemotherapy cycles were proposed. These are the protocol treatments, but there could be some variation in the modality and duration of these treatments.
Statistical analysis
A range of analyses has been performed. We have studied the frequencies of the demographic and clinical variables, and the Quality of Life scores. We have performed a known-groups comparison with one way analysis of variance (ANOVA). If the homoscedasticity test was unsatisfactory (i.e. the Levene score was lower than 0.05) we have employed the Welch statistic to compare means. At the first assessment, we carried out analyses with the core questionnaire and the part of the lung module that assesses the symptoms of the disease (the pain and dyspnoea scales) and the items of haemoptysis and coughing, and have compared subgroups based on local/advanced disease stages. At the second assessment, we used the core questionnaire and those parts of the lung module that assess side effects (hair loss, sore mouth, trouble swallowing and neuropathy), and have compared subgroups based on treatment modality (chemotherapy vs. combined treatment). Changes in Quality of Life scores over time were calculated and tested for statistical significance by means of repeated measures analyses of variance. We have compared scores in the core and the lung cancer questionnaires at the three time points using T-tests for related samples to establish between which pairs of the three assessments the significant differences appeared. In all the analysis performed in the present study, a p value of <0.05 is considered statistically significant.
Results
169 patients completed the baseline questionnaire, with 90% fitting the inclusion criteria. 17 patients refused to participate in the study; these were at an advanced stage of the disease (small cell lung cancer with advanced disease and non-small cell stage IV lung cancer). 115 patients also completed the second assessment and 42 the third. The reasons for not completing the second and third measures were a reduction in the proposed treatment as a consequence of patients' physical status, and death in a few cases. All completed questionnaires included answers to more than 70% of the items. The demographic and clinical characteristics of the sample are presented in Table II. There are more patients with limited disease in this sample. On performance status, moderately high values at the three assessments predominate. More than half of the sample showed no weight loss. The treatment modality that is administered most often is chemotherapy, either alone or combined with chest radiotherapy. Four patients with non-small cell stage IV disease received radiotherapy alone with palliative intention, and completed just the first assessment given their disease progression and poor physical status. Toxicity scores are higher at the second than at the third assessment.
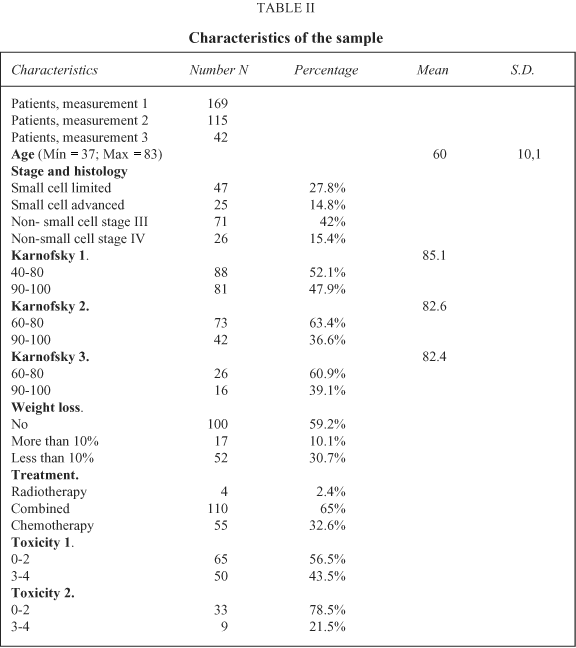
Quality of Life scores at the pre-treatment assessment show high levels of limitations in the Global Quality of Life and role functioning scales, and in the symptoms of fatigue, coughing, sleep disturbance, dyspnoea and appetite loss. Minor problems were evident on the pain scale. At the second and third time points, there were similar limitations, and also in the neuropathy and hair loss items (Tables III and IV).
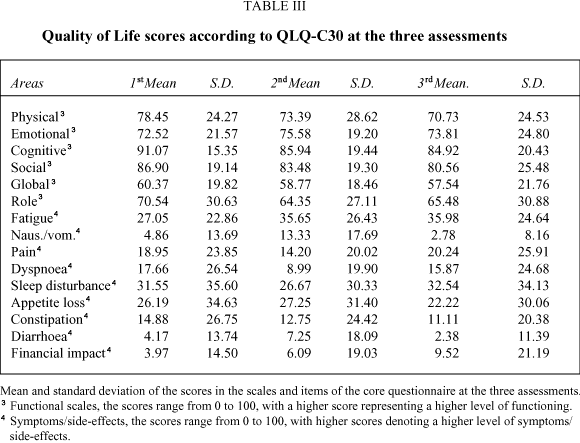

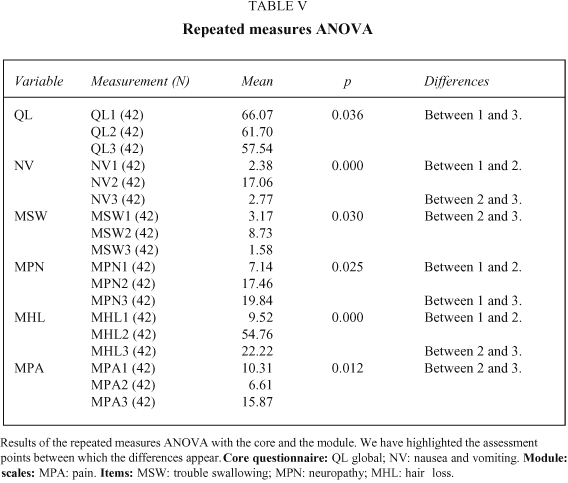
The group comparisons performed at the first assessment, with disease stage as the grouping variable, show significant differences on the pain and global scales of the core and in the pain scale of the module, with a better Quality of Life in the limited disease stages. In the analysis performed at the second measurement, with the variable treatment modality, there is a significant difference in the core dyspnoea item, with higher levels of this symptom in the chemotherapy group.
The repeated measures analysis of variance shows significant differences within the core questionnaire in the global (with a higher Quality of Life at the third than the first assessment) and the nausea and vomiting scales (with more toxicity at the second than the first and third time points). In the module there are significant differences on the pain scale (more pain at the third than the second time point) and the items of neuropathy (less toxicity at the first than at the second and third assessments), hair loss (greater at the second time point than at the third and first), and trouble swallowing (more trouble at the second than at the third time point; see Table VI).
Discussion
In this paper we present the results of a quality of life study carried out with a sample of lung cancer patients at different stages of disease. One aspect that we wish to highlight is the high degree of collaboration between patients and professionals in order to participate in this Quality of Life study. We have had a high rate of compliance at the first assessment, which is important if we compare it with other Quality of Life studies. The clinical and demographic data of this sample are representative of lung cancer patients in our setting. The reduction in the number of patients who have completed the second and third assessments is common in lung cancer studies34.
Performance status at the first assessment is in the middle-high range and weight loss scores were low, indicating that the treatment is proposed initially to patients who can adequately stand it. Performance status is also in the middle-high level and the toxicity scores moderate at the second and third time points indicating that, during the different treatment phases, chemo-/radiotherapy are proposed only to patients who can deal with it adequately. One month after the end of treatment there is moderate toxicity which, in the case of hair loss, could improve and, in the case of neuropathy, could last longer. The proportion of patients who receive chemotherapy is representative of the treatment modalities that are administered in our setting and could be different at other centres.
The Quality of Life questionnaires show rather low scores at the three assessments in areas that can be related to lung cancer, such as role functioning, symptoms of the disease and also global Quality of Life. There is a low level of pain indicating that there is good control of this symptom. Sleep disturbance has an emotional basis. At the second and third time points, there are moderately low scores in areas of treatment secondary effects like neuropathy, hair loss and others. These Quality of Life scores also support the view that patients who receive treatment are able to withstand it adequately.
There are few differences in Quality of Life between the different disease stages, which may be a consequence of the inclusion of only patients with un-resectable disease. There are also few differences in the analysis with treatment modalities, given that there are no patients with radiotherapy as a unique treatment at the second and third assessment. The results of the repeated measures analysis of variance in Quality of Life areas that are common to other cancer sites and disease symptoms show that, during the treatment and follow-up periods, the Quality of Life of this sample of lung cancer patients is stable, shows a moderate decrease on some dimensions, but has not improved in any area. A poorer level in the global scale at the third assessment compared to the first one could be a consequence of the evolution of the disease and the burden of treatment. The higher level of pain at the third than the second measurement could also be a consequence of the progression of the disease. These results, including the lack of improvement in these quality of life areas, underscore the severity of the disease and the limitations of treatments. The scope of these limitations could be greater if we take into account that there are groups of patients who have not followed the treatment. It would be interesting to compare these results with the Quality of Life scores of patients who have not completed the protocols proposed.
Some of the secondary effects assessed with the Quality of Life questionnaires increase during the treatment and, in the follow-up period, the more transient effects such as nausea, vomiting, trouble swallowing and hair loss decrease and the more endurable toxic effects, like neuropathy, persist. These results are similar to the toxicity values reported by the physicians, and might be a consequence of the cisplatin-based treatment regime.
In conclusion, the quality of life of the patients in this sample who have completed the questionnaires at the different assessment points is moderately high, showing few differences between groups based on stage of disease or treatment modality. Quality of life scores during treatment were stable in most areas. There was some worsening in areas related to treatment secondary effects that, in some cases, improved in the follow-up period, and in global quality of life and pain, and no improvements in any area.
Acknowledgements
We would like to acknowledge the help of the Oncology Departments of the Hospital of Navarre.
This work has received the support of a grant from the Health Department of the Gobierno de Navarra.
References
1. Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small cell lung cancer: how much benefit is enough? J Clin Oncol 1993; 11: 1866-1872. [ Links ]
2. Herndon JE, Fleishman S, Kornblith AB, Kosty M, Green MR, Holland J. Is quality of life predictive of the survival of patients with advanced non-small cell lung carcinoma? Cancer 1999; 85: 333-340. [ Links ]
3. Helsing M, Bergman B, Thaning L, Hero U. Quality of Life and survival in patients with advanced non-small cell lung cancer receiving supportive care plus chemotherapy plus carboplatin and etoposide or supportive care only. A multicentre randomised phase III trial. Eur J Cancer 1998; 34: 1036-1044. [ Links ]
4. Hürny C, Bernhard J, Joss R et al. Feasibility of Quality of Life assessment in a randomized phase III trial of small cell lung cancer. A lesson from the real world. The Swiss Group for Clinical Cancer Research SAKK. Ann Oncol 1992; 3: 825-831. [ Links ]
5. Gower NH, Rudd RM, Ruiz de Elvira MC et al. Assessment of Quality of Life using a daily diary card in a randomised trial of chemotherapy in small cell lung cancer. Ann Oncol 1995; 6: 575-580. [ Links ]
6. Joss RA, Alberto P, Hürny C et al. Quality versus quantity of life in the treatment of patients with advanced small cell lung cancer? A randomized phase III comparison of weekly carboplatin and teniposide versus cisplatin, adriamycin, etoposide alternating with cyclophosphamide, methotrexate, vincristine and lomustine. The Swiss Group for Clinical Cancer Research SAKK. Ann Oncol 1995; 6: 41-48. [ Links ]
7. Giaconne G, Splinter TA, Debruyne C et al. Randomized study of paclitaxel-cisplatin versus cisplatin-teniposide in patients with advanced non-small cell lung cancer. The European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1998; 16: 2133-2141. [ Links ]
8. Sundstrom S, Bremnes R, Aasebo U et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 2004; 22(5):801-180. [ Links ]
9. Mantovani G, Astara G, Manca G et al. Endoscopic laser ablation as palliative treatment of endobronchial, nonresectable, or recurrent lung cancer: assessment of its impact on quality of life. Clin Lung Cancer 2000; 1(4):277-285 [ Links ]
10. Langendijk H, de Jong J, Tjwa M et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol 2001; 58(3):257-268. [ Links ]
11. Smith IE, O'Brien ME, Talbot DC et al. Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol 2001; 1;19(5):1336-1343. [ Links ]
12. Aaronson NK, Ahmezdai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a Quality of Life instrument for use in intentional clinical trials. J Natl Cancer Inst 1993; 85: 365-376. [ Links ]
13. Aaronson NK, Cull A, Kaasa S, Sprangers M. The EORTC modular approach to Quality of Life assessment in Oncology. Int J Ment Health 1994; 23: 75-96. [ Links ]
14. Kiebert GM, Kaasa S. Quality of Life in clinical trials: experience and perspective of the European Organization for Research and Treatment of Cancer. J Natl Cancer Inst 1993; 20: 91-95. [ Links ]
15. Sprangers MAG, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer approach to Quality of Life assessment: guidelines for developing questionnaire modules. Qual Life Res 1993; 2: 287-295. [ Links ]
16. Sprangers M, Groenvold M, Arraras JI et al. The European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life questionnaire module: first results from a three-country field study. J Clin Oncol 1996; 14: 2756-2768. [ Links ]
17. Arraras JI, Arias F, Tejedor M et al. El cuestionario de Calidad de Vida para tumores de cabeza y cuello de la EORTC QLQ-H&N35. Estudio de validación para nuestro país. Oncología 2001; 24(10): 482-491. [ Links ]
18. Arraras JI, Vera R, Manterola A et al. El cuestionario de Calidad de Vida para cáncer colorectal EORTC QLQ-CR38. Estudio de validación para nuestro país. Oncología 2003; 26 (9): 285-292 [ Links ]
19. Bjordal K, de Graeff A, Fayers PM et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur J Cancer 2000; 36:1796-1807. [ Links ]
20. Kaasa S, Bjordal K, Aaronson NK et al. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer 1995; 31A: 2260-2263. [ Links ]
21. Porzsolt F, Wolpl CP, Rist CE, Kosa R, Buchele G, Gaus W. Comparison of 3 instruments (QLQ-C30, SF-36, QWB-7) measuring health related quality of life quality of well-being. Psych Oncol 1996; 5: 103-117. [ Links ]
22. Zieren HU, Muller JM, Hamberger U, Pichlmaier H. Quality of Life after surgical therapy of bronchogenic carcinoma. Eur J Cardiothorac Surg 1996; 10: 233-237. [ Links ]
23. McMillan DC, Wigmore SJ, Fearon KC, O'Gorman P, Wright CE, McArdle CS. The use of megestrol acetate in the treatment of weight loss in gastrointestinal cancer patients with weight loss. Br J Cancer 1999; 79: 495-500. [ Links ]
24. Bergman B, Aaronson NK, Ahmezdai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC core Quality of Life questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer 1994; 30A: 635-642. [ Links ]
25. Montazeri A, Gillis CR, McEwen J. Quality of Life in patients with lung cancer. A review of literature from 1970 to 1995. Chest 1998; 113: 467-481. [ Links ]
26. Gridelli C, Perrone F, Nelli F et al Quality of life in lung cancer patients. Ann Oncol 2001;12 Suppl 3:S21-25. [ Links ]
27. Arraras JI, Tejedor M, Arias F et al. El Grupo de Calidad de Vida de la EORTC. Estudios del Servicio de Oncología del Hospital de Navarra dentro de este organismo. Oncología 2000; 23(6): 302-306 [ Links ]
28. Folstein M, McHugh PR. Mini-mental state: a practical method for adding the cognitive state of patients for the clinician. J Psy Res 1975; 12: 189-198. [ Links ]
29. Arraras JI, Pruja E, Tejedor M, Illarramendi JJ, Dominguez MA, Valerdi JJ. El cuestionario de Calidad de Vida de la EORTC, QLQ-C30 (versión 2.0). Estudio estadístico de validación para nuestro país con pacientes con cáncer de pulmón. Rev Oncología 1999; 1: 257-263. [ Links ]
30. Arraras JI, Pruja E, Marcos M et al. El cuestionario de Calidad de Vida para cáncer de pulmón de la EORTC QLQ-LC13. Estudio de validación para nuestro país. Oncología 2000; 23: 127-134. [ Links ]
31. Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948; 1: 634-656. [ Links ]
32. NCI Common toxicity criteria. National Cancer Institute, Division of Cancer Treatment, 1988. [ Links ]
33. Valerdi JJ, Tejedor M, Molina F et al. Combined treatment with induction chemotherapy and hyperfractionated radiotherapy for the treatment of stage III non-small cell lung cancer. Proc ASCO 1996; 408. [ Links ]
34. Hopwood P, Harvey A, Davies J et al. Survey of the administration of Quality of Life questionnaires in three multicentre randomised trials in cancer. Eur J Cancer 1998; 34: 49-59. [ Links ]
 Correspondence to
Correspondence to
Dr. J. I. Arraras
Hospital de Navarra
Servicio de Oncología
Irunlarrea, 3 E-31008 Pamplona
E-mail: jiarraras@correo.cop.es
Recibido: 14.12.04
Aceptado: 20.12.04
# Este trabajo ha contado con la ayuda de una beca del Departamento de Salud del Gobierno de Navarra.














