Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.3 Madrid mar. 2004
| POINT OF VIEW |
Antiviral therapy of recurrent hepatitis C in liver transplantation
I. Fernández and C. Loinaz1
Service of Digestive Diseases and 1Unit of Abdominal Transplants. Hospital 12 de Octubre. Madrid, Spain
Fernández I, Loinaz C. Antiviral therapy of recurrent hepatitis C in liver transplantation. Rev Esp Enferm Dig 2004; 96: 201-214.
Recibido: 15-01-04.
Aceptado: 17-02-04.
Correspondencia: Inmaculada Fernández Vázquez. Servicio de Aparato Digestivo. Hospital 12 de Octubre. Avda. de Córdoba, s/n. 28045 Madrid. Telf.: 91 390 84 09 - Fax: 91 390 82 80. e-mail: ifernandez@medynet.com
Cirrhosis secondary to hepatitis C virus (HCV) infection has become the primary indication for liver transplantation (LT) both in Europe and the USA. In our setting, it is responsible for more than 40% of LTs. HCV infection relapse following transplantation is an ubiquitous fact, and histologically damages the graft in most patients during follow-up (2,3). Despite excellent initial results regarding survival in transplant recipients for cirrhosis and HCV infection (4-6), recent studies revealed that the natural history of recurrent hepatitis C accelerates following transplantation. There is a progression to cirrhosis in up to 30% of patients within 5 years (7), and many of these patients experience their first clinical decompensation within one year after diagnosis (8). On the other hand, 2-8% experience early graft failure as a result of the development of severe cholestatic hepatitis -the so-called fibrosing cholestatic hepatitis- without cirrhosis (9). This progression of the graft's histologic lesion in relation to HCV infection may help explain the fact that decreased survival was observed in a number of centers for both the graft and patient in individuals with HCV infection, versus non-infected individuals with LT (10,11).
However, the natural history of recurrent hepatitis C is variable, and up to 30% of patients remain free of liver cirrhosis at 5 years after transplantation (12). Various factors have been involved in aggressive post-transplant hepatitis C. The increasingly powerful immunosuppressive therapy given to these patients stands out because of its relevance and modification potential (13). Recently increased donor age has also been related to poor recurrent hepatitis C outcomes (14). Both factors may help explain the fact that patients receiving transplantation after 1995-1996 exhibit a faster progression to liver cirrhosis when compared to those transplanted earlier (3).
Antiviral therapy for recurrent hepatitis C in LT is a controversial subject. Considering the natural history of infection following transplantation, this is clearly a therapeutic option that should be considered for transplanted patients with chronic hepatitis C with the aim of delaying the progression of liver injury and thus preventing graft failure. However, the currently available therapy, consisting of interferon (IFN) in combination with ribavirin, has serious shortcomings: overall efficacy is lower and tolerability is poorer whe compared to non-transplanted patients with chronic hepatitis C. Patients with LT and recurrent hepatitis C have several predictors of poor response to antiviral therapy, which include high prevalence of infection by HCV genotype 1b (2,15,16), high viral load (17,18), and need of immunosuppressive therapy, which may inhibit virologic response to interferon (19). On the other hand, these patients commonly present with severe medication side effects amongst which hemolytic anemia and neutropenia are of particular relevance, as doses must be reduced down to a subtherapeutic level or even complete discontinuation.
HCV infection in LT may be also managed either in the immediate post-transplant period, with the aim of preventing the development of chronic hepatitis in the graft, or prior to transplantation, to prevent graft infection. Currently available data provide no insight into which therapeutic strategy is best. Most reports refer to small pilot studies having different inclusion criteria and efficacy definitions, while randomized, controlled studies are few.
TREATMENT OF HEPATITIS C BEFORE TRANSPLANTATION
Antiviral therapy may be prophylactically used during the pre-transplantation period, with the aim of modifying the outcome of hepatitis C in the post-transplantation period. Transplant recipients with high HCV levels before LT have been shown to experience mortality and graft loss rates that are 30% higher than those of recipients with low viral load (20). Interferon alone or in combination with ribavirin has been shown to reduce HCV RNA titers in patients with cirrhosis (21,22). However, a major portion of these transplant-waiting patients do not tolerate therapeutic doses of antiviral medication, liver decompensation may worsen, and medication side effects may be serious, particularly in terms of severe cytopenias and increased infection (21,22). With regard to these shortcomings, the use of titrated doses of interferon and ribavirin over weeks or months, and the use hematologic growth factors to prevent severe anemia and neutropenia, managed to make cirrhotic patients with stable liver function (intermediate Child-Pugh grade - 7.1) able to tolerate medication in a study by Everson et al (23). In a paper recently published by Forns et al 9 of 30 LT waiting list patients with HCV-related cirrhosis who received interferon and ribavirin underwent viral negativization during therapy (24). Of these 9 patients, 6 remained HCV infection-free after LT. Treatment was initiated when the expected mean time to transplantation was 4 months. The incidence of side effects was common and led to drug discontinuation in 6 patients and treatment change in 18 patients.
According to cumulative experience, the first consensus meeting on LT and HCV, which was held by ILTS (International Liver Transplantation Society) on March 2003, issued guidelines to select patients with cirrhosis and HCV infection in LT waiting lists to receive interferon and ribavirin: patients with a Child-Pugh index below or equal to 7, or MELD index smaller than or equal to 18 will be eligible for treatment. Treatment will be proposed to only thoroughly selected cases with above-mentioned indices of 8-11 or 18-25, respectively, and therapy will be avoided for scores above 11 or 25 (25). Patients infected by HCV genotypes 2 and 3 will be carefully assessed, as their possibilities of sustained viral response are high. According to this consensus meeting, maintenance treatment may be considered for patients who relapse after therapy discontinuation, to clear away infection or reduce viremia until transplantation time.
TREATMENT OF HEPATITIS C AFTER TRANSPLANTATION
Antiviral therapy in the early post-transplant period (preemptive therapy)
The goal of antiviral therapy during the early post-transplant period is to reduce the incidence and/or severity of recurrent hepatitis C. This action considers that the graft is being "acutely" infected by HCV. Studies on viral kynetics show minimal HCV blood levels during the anhepatic phase and immediate postoperative period (26). As of the second week post-LT, viral load increases in all patients, and peaks between months one and three post-transplant. Antiviral therapy administration within the first month after transplantation is based on the fact that transplantation has been shown to consistently eliminate viruses in more than 90% of immunocompetent patients with acute hepatitis C (27), a percentage higher than that seen in patients with chronic hepatitis (28,29).
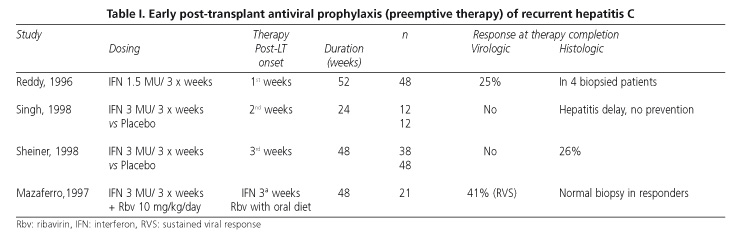
Reports evaluating the prophylactic use of antiviral therapy to prevent recurrent hepatitis C are few, and no comparative studies exist on the efficacy and safety of antiviral therapy during the early post-transplant period versus established, recurrent hepatitis C with obvious histologic damage. Three studies with IFN-α a in monotherapy and one study with IFN-α in combination with ribavirin have been published to this day (Table I). In a non-controlled study by Reddy et al (30), 48 patients received IFN in the week following transplantation for 52 weeks. Upon therapy completion, viremia was undetectable in one third of patients, and liver biopsy revealed no chronic hepatitis in four patients. In this study, IFN was well tolerated and did not increase the incidence of rejection. Singh et al (31) reported on a controlled study in which 24 patients were randomized to receive either IFN or placebo for 6 months, starting in the second week following transplantation. Although the incidence and severity of histologic recurrence did not differ between both groups, a delayed development of hepatitis C was seen in patients who received IFN (408 vs 193 days, p = 0.05). However, no increased survival was demonstrated for grafts or patients in the IFN group after a mean follow-up of 28 months.
In a second controlled study (32), 86 patients transplanted for cirrhosis and HCV infection were randomized to receive IFN in week 2 following transplantation (n = 38) or placebo (n = 48) for 1 year. Although the rate of viral persistence was not affected by treatment, the histologic recurrence of disease at one year after transplantation was less common in the IFN group (8 of 30 evaluated patients) versus non-treated individuals (22 of 41 evaluated patients, p = 0.01). Graft and patient survival, however, did not differ between both groups at 2 years after transplantation. Side effects were common, particularly leukopenia. Also in this study, no increased incidence of rejection was observed in the IFN group. Interestingly, IFN reduced the incidence of recurrent hepatitis C without modifying viremia levels, which suggests it may act via a mechanism differing from that of its antiviral effect.
Mazzaferro et al (33) assessed the efficacy of a combination therapy using IFN-α 2b and ribavirin to prevent recurrent hepatitis C. They carried out a non-controlled study with 21 patients who received IFN and ribavirin for 48 weeks, starting in the third week after transplantation. After one year of follow-up, only 1 of 21 patients (5%) had active chronic hepatitis, and 4 (19%) had evidence of acute hepatitis. In 9 patients (41%) no HCV RNA was detected in the serum or liver biopsy samples. Of these 21 patients, 11 (52%) remained with detectable viremia but free of disease progression. No patient discontinued medication because of toxicity or graft rejection. According to these results, the natural history of recurrent hepatitis C may significantly improve by initiating IFN-α and ribavirin during the immediate post-transplant period. However, these results should be confirmed. On the other hand, the use of the renally-cleared ribavirin may be expected to raise difficulties in many patients, bearing in mind that the majority of them exhibit renal dysfunction during this period.
Pegilated interferons alpha 2a and alpha 2b have been recently incorporated into the therapeutic armamentarium against hepatitis C. Using pegylation, blood levels -already stable for hours with standard IFN-α reach a steady state for several days with pegynterferon. These drugs have been shown to achieve sustained viral response in a higher number of patients (39%) when compared to non-pegylated alpha IFN (19%) (34,35). Preliminary results from a study using pegylated interferon α-2a at a dose of 180 µg per week for 48 weeks, starting in week three after LT, showed that 22% of treated patients achieved viral negativization at week 24, versus 0% in the control group (36).
Considering reported data, the first ILTS consensus meeting suggested that antiviral therapy be used in the immediate post-transplant period only under exceptional circumstances such as retransplantation, until future studies define its actual usefulness in the prevention of recurrent hepatitis C (25).
Immunoprophylaxis of hepatitis C in liver transplantation
In 1998, retrospective study showed that polyclonal gammaglobulin administration containing ant-HCV may reduce HCV viral load in transplanted patients with hepatitis C (37). However, a controled study analyzed the efficacy of anti-HCV immune globulin in preventing the recurrence of HCV infection following LT, with unsatisfactory results (38). In this work, 26 patients were randomized to receive either high immunoglobulin doses, low immunoglobulin doses, or placebo during the anhepatic phase. The administration of immune globulin modified neither the clinical nor the virological outcome for treated patients. Therefore, the administration of anti-HCV immunoglobulin to transplanted patients is currently discouraged.
Antiviral therapy of recurrent hepatitis C
The treatment of post-LT recurrent hepatitis C is based on cumulative experience with the treatment of chronic hepatitis C in non-transplanted patients. Safety and potential drug interactions are particularly relevant factors besides effectiveness within the context of LT.
Interferon
A number of studies have assessed the effectiveness of IFN in the treatment of post-LT hepatitis C with unsatisfactory results (40-44) (Table II). Despite the presence of virological response reaching 50% at therapy completion in one study (43), the reappearance of viremia upon IFN discontinuation was a universal fact in all of them. Similarly, biochemical response was transient and histology did not improve following therapy in most studies. The most commonly used regimen was 3 MU IFN-α 3 times per week for 6 months. Except for one study where patients had acute hepatitis (42), IFN therapy was never set on before three months after transplantation.
Wright et al saw transaminase levels returning to normal values in 5 (28%) of 18 treated patients (40). Serum viral RNA decreased in all cases regardless of transaminase levels, but only became negative in a patient who relapsed upon medication withdrawal. Nor did IFN significantly modify liver histology. In this study, responders were defined as patients whose transaminase levels had returned to normal at treatment completion. This group had pre-treatment viral loads and bilirubin levels that were significantly lower than those in the non-responder group, as well as longer lapses from transplantation time to IFN therapy onset.
Two subsequent works showed no benefits for IFN therapy regarding biochemical activity, viral replication inhibition, and liver injury in transplanted patients with recurrent hepatitis C (41,42). Of late, Cotler et al (44) used IFN-α at doses of 3 MU daily, and reported therapy completion virological response in one half of treated patients. However, only one patient had a sustained response upon medication withdrawal, this being the only one infected by a genotype other than 1, namely genotype 3.
Although the efficacy of IFN monotherapy for recurrent hepatitis C has been proven scarce, safety is a major concern regarding its use in LT, as it may induce acute or chronic rejection. This has been commonly seen in renally transplanted patients (39). In this respect, results are conflicting for LT. In two of the aforementioned studies IFN caused rejection in a considerable percentage of patients (41,42). Feray et al observed chronic rejection in 5 of 14 treated patients, and only in 1 of 32 patients with recurrent hepatitis C who did not receive IFN (41). However, IFN did not increase rejection possibilities in the remaining studies (40,43,44).
Ribavirin
Ribavirin, an analog of guanosine, has a powerful antiviral activity against a wide variety of both RNA and DNA viruses. In immunocompetent patients with chronic hepatitis C, ribavirin monotherapy normalizes transaminase levels in 30-40% of cases, and reduces lobular inflammation rates. However, biochemical response is transient, no inhibition of viral replication occurs, portal and periportal inflammation are left unchanged, and progression to liver fibrosis remains unarrested (45,46).
Studies assessing ribavirin efficacy in patients withchronic hepatitis C following LT are few, and results are similar to those obtained in the non-transplanted population (43,47,48) (Table III). In the study by Gane et al (43), 13 (93%) of 14 patients who received ribavirin for 6 months had their transaminase levels normalized, but serum viral RNA remained detectable in all cases. Lobular inflammation decreased following therapy in 64% of patients; however, the total histological inflammatory activity index remained unchanged in all patients. In another study 18 patients were treated with ribavirin for an average of 23 months (12-44 months) (48). Although the infection's biochemical activity decreased in 69% of cases, and transaminases went back to normal in 28% of cases, long-term therapy using ribavirin did not manage to prevent progression to liver fibrosis either. Furthermore, histologic inflammatory activity and fibrosis indices worsened after ribavirin treatment in two recently reported pilot studies, in spite of decreased transaminase levels (49,50).
The most relevant side effect of ribavirin was hemolytic anemia. In some reports, up to 50% of treated patients experienced hemoglobin reductions below 10 g/dL (33). From our experience, this is a virtually universal side effect in transplanted patients, which demands that hemoglobin levels be closely monitored during treatment. Transplanted patients are more susceptible of suffering from hemolytic anemia when compared to non-transplanted individuals. This fact may well be related to decreased creatinine clearance, a common finding post-transplant that is mainly due to cyclosporin or tacrolimus renal toxicity. On the other hand, both drugs may accumulate in red blood cells and thus increase ribavirin-induced cell membrane fragility (51,52).
Alpha-interferon and ribavirin
In immunocompetent patients with chronic hepatitis C the addition of ribavirin to interferon increased sustained response rates. Various multicenter, randomized, controlled studies showed that combined IFN-α and ribavirin elicited sustained viral responses in 28-40% of these patients, or at least twice as many responses as those seen with IFN monotherapy (53,54). Such response is primarily conditioned by HCV genotype, and is nearly 80% when only patients infected by genotypes 2 and 3 are evaluated.
Since 1997 several centers reported their experience with using interferon and ribavirin in the treatment of liver transplant recipients with established hepatitis C (55-63) (Table IV). However, definitive conclusions are difficult to draw from currently available data. Most studies are non-controlled pilot studies with heterogeneous inclusion criteria. In some of them, only patients with severe hepatitis C recurrence were selected. Their common primary goal -sustained viral response- was achieved in 5-30% of patients using an intention-to-treat analysis. When biochemical response at therapy completion was evaluated, results obtained were disparate and ranged from 18% in some study to 100% in the first report by Bizollon et al in 1997 (55). With the exception of two studies (58,62) where improved histologic activity was seen in patients whose transaminase levels returned to normal values at therapy completion, combined treatment using interferon and ribavirin did not improve liver histology in the rest. Liver biopsy had been performed at therapy completion in these patients. Of late, Bizollon et al (64) showed obvious histological improvement in 13 (86%) of 14 patients with sustained viral response, who underwent a liver biopsy at 3 years after treatment completion. Inflammatory activity was decreased and fibrosis had not progressed at 3 years following therapy discontinuation.
Samuel et al (63) recently reported on a controlled study where 42 patients were randomized to receive IFN-α 2b (3 MU/3 times per week) and ribavirin (1000-1200 mg/d) (n = 28) or placebo (n = 24) for one year. They observed a virological response in 32% of patients in the combination regimen group at therapy completion, and in 21.4% of them at 6 months after therapy completion. However, viremia became negative in none of the untreated patients during the study (p = 0.036).
The optimal duration of combined therapy is un-known. The most commonly used regimen was IFN-α at a dose of 3 MU three times per week, and ribavirin 800-1200 mg/d for 12 months. In a recent study (62), however, no significant differences were seen between 6-month and 12-month regimens. On the other hand, it has been suggested that maintenance treatment with ribavirin after combined therapy completion may improve response (55,58). Furthermore, some authors have used combined maintenance therapy to prevent relapse in patients with no virological response after 12 months of interferon and ribavirin treatment (65). Controlled studies are needed to define optimal therapy duration for these patients.
Although predictive factors of response are not well established, it seems obvious that the severity of recurrent hepatitis C plays a major role in the response to antiviral therapy. Cases reported on patients with severe post-LT cholestatic hepatitis C who received interferon and ribavirin show overall discouraging results (66,67). Some authors have recommended indefinite treatment with interferon and ribavirin as the only feasible therapeutic strategy for these patients, given the universal, always severe recurrence of disease seen in their patients upon therapy discontinuation (68).
Side effects represent a major shortcoming of combination therapy. In the aforementioned studies, one third of transplanted patients failed to complete their therapy on average, which obviously limits the effectiveness of medication in this population. The most severe side effect of IFN is neutropenia secondary to bone marrow depression. This effect is even more serious in transplanted patients, since they receive other myelosuppressive drugs such as immunosuppressants, antiviral agents or antibiotics, which may exacerbate both neutropenia and their potential risk for infection. The use of granulocyte colony-stimulating factor (GCSF) has proven useful in the treatment of severe IFN-αinduced neutropenia (69); although it was never shown to increase the effectiveness of combination therapy, its use has become increasingly common for transplanted patients. The administration of ribavirin entails even greater risks in the setting of LT. In reported studies 50-90% of patients required dose reductions, and almost one half definite discontinuation. The need for transfusion for severe anemia was not an exceptional fact. Similarly, most studies used erythropoietin to keep ribavirin at therapeutic doses, even though the former's effectiveness was never assessed regarding antiviral therapy outcome. Dose titration is recommended for this antiviral agent, particularly when mild renal failure is present. Its use is contraindicated in patients with moderate to severe renal failure.
Pegylated interferon and ribavirin
Pegylated interferon α-2a or 2b in combination with ribavirin is currently the treatment of choice for chronic hepatitis C in immunocompetent patients (70,71). Its efficacy -measured as sustained viral response- approaches 60%; this percentage goes up to 80% in patients infected by genotypes 2 and 3, and down to below 43% in patients infected by genotype 1. Preliminary results for transplanted patients -still as oral communications in meetings- show noticeably poorer results (72,73): sustained viral response was attained in 26-36% of patients, most of them infected by genotype 1. In these studies, adverse events still were the primary shortcoming of this medication.
CONCLUSIONS
The outcome of recurrent hepatitis C in LT is a matter of increasing concern. Retransplantation results in these patients are poor (74), which together with an increasing relative scarcity of donors makes this option an increasingly remote possibility in most LT programs. Therefore, a search for strategies capable of changing the natural history of hepatitis C after transplantation is needed. On the one hand, an improved control of factors that may accelerate progression is essential; on the other hand, an optimal antiviral therapy for this condition must be found. In waiting for novel antiviral agents, pegylated interferons combined with ribavirin represent the best therapeutic option that is currently available. A number of essential questions remain unsolved: when and whom to treat, and for how long? Studies comparing the efficacy and safety of antiviral therapy at different transplantation times are needed to select patients with actual response possibilities and to define the best therapeutic scheme for this population.
REFERENCES
1. Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology 1992; 103: 317-22. [ Links ]
2. Feray C, Caccamo L, Alexander G, Ducot B, Gugenheim J, Casanovas T, et al. HCV and liver transplantation: Preliminary results of a European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. Gastroenterology 1999; 117: 619-25. [ Links ]
3. Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, et al. HCV-related fibrosis progression following liver transplantation: Increase in recent years. J Hepatol 2000; 32: 673-84. [ Links ]
4. Gane E, Portmann B, Naoumov N, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996; 334: 815-20. [ Links ]
5. Lumbreras C, Colina F, Loinaz C, Domingo MJ, Fuertes A, Dominguez P, et al. Clinical, virological, and histologic evolution of hepatitis C virus infection in liver transplant recipients. Clin Infect Dis. 1998; 26: 48-55. [ Links ]
6. Feray C, Gigou M, Samuel D, Paradis V, Wilbert J, David MD, et al. The course of hepatitis C virus infection after liver transplantation. Hepatology 1994; 20: 1137-43. [ Links ]
7. Prieto M, Berenguer M, Rayón JM, Córdoba J, Argüello L, Carrasco D, et al. High incidence of allograft cirrosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology 1999; 29: 250-6. [ Links ]
8. Berenguer M, Prieto M, Rayon J, Mora J, Pastro M, Ortiz V, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology 2000; 32: 852-8. [ Links ]
9. Schluger L, Sheiner P, Thung S, Lau J, Min A, Wolf D, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 1996; 23: 971-6. [ Links ]
10. Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucy MR. Association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002; 122: 889-96. [ Links ]
11. Sanchez-Fueyos A, Restrepo JC, Quintó L, Bruguera M, Grande L, Sanchez-Tapias JM, et al. Impact of recurrence of HCV infection after liver transplantation on the long-term viability of the graft. Transplantation 2002; 73: 56-63. [ Links ]
12. Berenguer M, Rayón M, Prieto M, Aguilera V, Nicolai D, Ortiz V, et al. Are posttransplantation protocol liver biopsies useful in the long-term? Liver Transpl 2001; 7: 790-6. [ Links ]
13. Papatheodoridis GV, Davies S, Dhillon AP, Teixeira R, Goulis J, Davidson B, et al. The role of different immunosupression in the long-term histological outcome of HCV reinfection after liver transplantation for HCV cirrhosis. Transplantation 2001; 72: 412-8. [ Links ]
14. Berenguer M, Prieto M, San Juan F, Rayón JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology 2002; 36: 202-10. [ Links ]
15. Feray C, Gigou M, Samuel D, Paradis V, Mishiro S, Maertens G, et al. Influence of genotypes of hepatitis C virus on the severity of recurrent liver disease after liver transplantation. Gastroenterology 1995; 108: 1088-96. [ Links ]
16. Ghobrial RM, Farmer DG, Baquerizo A, Colqhoun S, Rosen HR, Yersiz H, et al. Orthotopic liver transplantation for hepatitis C: outcome, effect of immunosuppression and causes of retransplantation during an 8-year single-center experience. Ann Surg 1999; 229: 824-31. [ Links ]
17. Chazouilleres O, Kim M, Coombs C, Ferrel L, Bachetti P, Roberts J, et al. Quantitation of HCV RNA in liver transplant recipients. Gastroenterology 1994; 106: 994-9. [ Links ]
18. Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann B, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology 1996; 110: 167-77. [ Links ]
19. Papatheodoridis GV, Barton SG, Andrew D, Clewley G, Davies S, Dhillon AP, et al. Longitudinal variation in hepatitis C virus (HCV) viraemia and early course of HCV infection after liver transplantation for HCV cirrhosis: The role of different immunosuppressive regimens. Gut 1999; 45: 427-34. [ Links ]
20. Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology 1998; 28: 823-30. [ Links ]
21. Crippin JS, Terrault N, McCashland TM, Sheiner PA, Charlton M. A pilot study of the torelability and efficacy of antiviral therapy in patients awaiting liver transplantation for hepatitis C (abstract). Hepatology 2000; 32:308A. [ Links ]
22. Everson GT, Trouillot T, Trotter J, Halprin A, McKinley C, Fey B, et al. Treatment of decompensated cirrhotics with a low-accelerating dose regimen (LADR) of interferon-alfa-2b plus ribavirin: safety and efficacy (abstract). Hepatology 2000; 32: 595A. [ Links ]
23. Everson GT, Trotter J, Euglenas M. Long-term outcome of patients with chronic hepatitis C and decompensatef liver disease treated with the LADR protocol (Low-Accelerating-Dose Regimen) (abstract). Hepatology 2002; 36: 297 A. [ Links ]
24. Forns X, Garcia-Retortillo M, Serrano T, Feliu A, de la Mata M, Suarez F, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol 2003; 39: 389-96. [ Links ]
25. Wiesner RH, Sorrell M, Villamil F and the ILTS Expert Panel. Report of the first international liver transplantation society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transplantation 2003; 9: S1-S9. [ Links ]
26. Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002; 35: 680-7. [ Links ]
27. Laeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med 2001; 345: 1452-7. [ Links ]
28. McHutchison JG, Gordon SC, Schiff ER, Schiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med 1998; 19: 1485-92. [ Links ]
29. Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med 1998; 19: 1493-9. [ Links ]
30. Reddy KR, Weppler D, Zervos XA, Nery JR, Webb MG, Khan MF, et al. Recurrent HCV infection following OLTx: the role of early post OLTx interferon treatment (abstract). Hepatology 1996; 24: 295A. [ Links ]
31. Singh N, Gayowski T, Wannstedt CF, Shakil AO, Wagener MM, Fung J, et al. Interferon-alpha for prophylaxis of recurrent viral hepatitis liver transplant recipients: a prospective, randomised, control trial. Transplantation 1998; 65: 82-6. [ Links ]
32. Sheiner PA, Boros P, Klion FM, Thung SN, Schluger LK, Lau J, et al. The efficacy of prophylactic interferon alfa-2b in preventing recurrent hepatitis C after liver transplantation. Hepatology 1998; 28: 831-8. [ Links ]
33. Mazzaferro V, Regalia E, Pulvirenti A, Tagger A, Andreola S, Pasquali D, et al. Prophylaxis againts HCV recurrence after liver transplantation. Effect of interferon and ribavirin combination. Transplant Proc 1997; 29: 519-21. [ Links ]
34. Lindsay K, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoef JC, et al. A randomized double-blind trial comparing pegylated interferon to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 2001; 34: 395-403. [ Links ]
35. Zeuzem S, Feinman V, Basenack J, Heathcote J, Lai M, Gane E, et al. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med 2000; 343: 1673-80. [ Links ]
36. Manzarbeitia C, Tepermann L, Chalasani N, Sheiner P, Wiesner R, Marks I, et al. 40 kDa peginterferon as prophylaxis against HCV recurrence after liver transplantation: preliminary results of a randomised, multicenter trial (abstract). Hepatology 2001; 34: 406A. [ Links ]
37. Feray C, Gigou M, Samuel D, Ducot B, Maisonneuve P, Reynes M, et al. Incidence of hepatitis C in patients receiving different preparations of hepatitis B immunoglobulins after liver transplantation. Ann Intern Med 1998; 128: 810-6. [ Links ]
38. Willems B, Ede M, Marotta P, Wall W, Greig P, Lilly L, et al. Anti-HCV human immunoglobulins for the prevention of graft infection in HCV-related liver transplantation, a pilot study. J Hepatol 2002; 36 (Supl.): 32. [ Links ]
39. Magnone M, Holley JL, Shapiro R, Scantlebury V, McCauley J, Jordan M, et al. Interferon-alpha induced acute renal allograft rejection. Transplantation 1995; 59: 1068-70. [ Links ]
40. Wright TL, Combs C, Kim M, Ferell L, Bachetti P, Ascher N, et al. Interferon alpha therapy for hepatitis C virus infection following liver transplantation. Hepatology 1994; 20: 773-9. [ Links ]
41. Feray C, Samuel D, Gigou M, Paradis V, David MF, Lemonnier C, et al. An open trial of interferon alpha recombinant for hepatitis C after liver transplantation: antiviral effects and risk of rejection. Hepatology 1995; 22: 1084-9. [ Links ]
42. Vargas V, Charco R, Castells L, Esteban R, Margarit C. Alpha-interferon for acute hepatitis in liver transplant patient. Transplant Proc 1995; 27: 1222-3. [ Links ]
43. Gane EJ, Lo SK, Riordan SM, Portmann BC, Lau JYN, Naoumov NV, et al. A randomized study comparing ribavirin and interferon alpha monotherapy for hepatitis C recurrence after liver transplantation. Hepatology 1998; 27: 1403-7. [ Links ]
44. Cotler SJ, Ganger DR, Kaur S, Rosenblate H, Jakate S, Sullivan DG, et al. Daily interferon therapy for hepatitis C virus infection in liver transplant recipients. Transplantation 2001; 71: 261-6. [ Links ]
45. Di Bisceglie AM, Conjeevaram HA, Fried MW, Sallie R, Park Y, Yurdaydin C, et al. Ribavirin as therapy for chronic hepatitis C. Ann Intern Med 1995; 123: 897-903. [ Links ]
46. Bodenheimer HC, Lindsay KL, Davis GL, Lewis JH, Thung SN, Mahaney K, et al. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: A multicentre trial. Hepatology 1997; 26: 473-7. [ Links ]
47. Aljumah AA, Cattral MS, Greig IR, Wanless IR, Krajden M, Hemming AW, et al. Long-term ribavirin therapy for recurrent hepatitis C after liver transplantation. Transplant Proc 1997: 29: 514. [ Links ]
48. Cattral MS, Hemming AW, Wanless IR, Al Ashgar H, Krajden M, Lilly L, et al. Outcome of long-term ribavirin therapy for recurrent hepatitis C after liver transplantation. Transplantation 1999; 67: 1277-80. [ Links ]
49. Quadri R, Giostra E, Roskams T, Pawlotsky JM, Mentha G, Rubbia-Brandt L, et al. Immunological and virological effects of ribavirin in hepatitis C after liver transplantation. Transplantation 2002; 73: 373-8. [ Links ]
50. Saab S, Hu R, Ibrahim AB, Goldstein LI, Kunder G, Durazo F, et al. Discordance between ALT values and fibrosis in liver transplant recipients treated with ribavirin for recurrent hepatitis C. Am J Transplant 2003; 3: 328-33. [ Links ]
51. Faure JL, Causse X, Bergeret A, Meyer F, Neidecker J, Paliard P. Cyclosporine induced haemolytic anemia in a liver transplant patient. Transplant Proc 1989; 21: 2242-3. [ Links ]
52. Winkler M, Schulze F, Jost U, Ringe B, Pichlmayr R. Anaemia associated with FK 506 immunosuppression. Lancet 1993; 341: 1035-6. [ Links ]
53. McHutchinson J, Gordon S, Schiff E, Schiffman M, Lee W, Rustgi V, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment of chronic hepatitis C. N Engl J Med 1998; 339: 1485-92. [ Links ]
54. Reichard O, Norkrans G, Fryden A, Braconier JH, Sonnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon a 2b with and without ribavirin for chronic hepatitis C. Lancet 1998; 351: 83-7. [ Links ]
55. Bizollon T, Palazzo U, Ducerf C, Chevallier M, Elliot M, Baulieux J, et al. Pilot study of the combination of interferon alfa and ribavirin as therapy of recurrent hepatitis C after liver transplantation. Hepatology 1997; 26: 500-4. [ Links ]
56. Götz G, Schön MR, Haefker A, Neuhaus T, Berg T, Hopt U, et al. Treament of recurrent hepatitis C virus infection after liver transplantation with interferon and ribavirin. Transplant Proc 1998: 30: 2104-6. [ Links ]
57. De Vera ME, Smallwood GA, Rosado K, Davis L, Martinez E, Sharma S, et al. Interferon-a and ribavirin for the treatment of recurrent hepatitis C after liver transplantation. Transplantation 2001; 71: 678-86. [ Links ]
58. Alberti AB, Belli LS, Airoldi A, de Carlis L, Rondinara G, Minola E, et al. Combined therapy with interferon and low-dose ribavirin in posttransplantation recurrent hepatitis C: a pragmatic study. Liver Transpl 2001; 7: 870-6. [ Links ]
59. Ahmad J, Dodson SF, Demetris AJ, Fung JJ, Shakil AO. Recurrent hepatitis C after liver transplantation: a nonrandomised trial of interferon alfa alone versus interferon alfa and ribavirin. Liver Transpl 2001; 7: 863-9. [ Links ]
60. Firpi RJ, Abdelmalek MF, Soldevila-Pico C, Reed A, Hemming A, Howard R, et al. Combination of interferon alfa-2b and ribavirin in liver transplant recipients with histological recurrent hepatitis C. Liver Transpl 2002; 8: 1000-6. [ Links ]
61. Shakil AO, McGuire B, Crippin J, Teperman L, Demetris AJ, Conjeevaram H, et al. A pilot study of interferon alfa and ribavirin combination in liver transplant recipients with recurrent hepatitis C. Hepatology 2002; 36: 1253-8. [ Links ]
62. Lavezzo B, Franchello A, Smedile A, David E, Barbui A, Torrani M, et al. Treatment of recurrent hepatitis C in liver transplants: efficacy of a six versus twelve month course of interferon alfa 2b with ribavirin. J Hepatol 2002: 37: 247-52. [ Links ]
63. Samuel D, Bizollon T, Feray C, Roche B, Si Ahmed SN, Lemonnier C, et al. Interferon-a 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology 2003; 124: 642-50. [ Links ]
64. Bizollon T, Ahmed SNS, Radenne S, Chevallier M, Chevallier P, Parvaz P, et al. Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut 2003; 52: 283-7. [ Links ]
65. Kornberg A, Hommann M, Tannapfel A, Grube Th, Schotte U, Scheele J. Combination therapy/prophylaxis with interferon and ribavirin for recurrent hepatitis C after liver transplantation: long-term effects on graft function and morphology. Transplant Proc 2002: 34: 2259-60. [ Links ]
66. Ong JP, Younossi ZM, Gramlich T, Goodman Z, Mayes J, Sarbah S, et al. Interferon a 2b and ribavirin in severe recurrent cholestatic hepatitis C. Transplantation 2001; 71: 1486-8. [ Links ]
67. Muramatsu S, Ku Y, Fukumoto, Iwasaki T, Tominaga M, Kusunoki N, et al. Successful rescue of severe recurrent hepatitis C with interferon and ribavirin in a liver transplant patient. Transplantation 2000; 69: 1956-8. [ Links ]
68. Gopal DV, Rosen HR. Duration of antiviral therapy for cholestatic HCV recurrence need to be indefinite. Liver Transpl 2003; 9: 348-53. [ Links ]
69. Colquhoun SD, Shaked A, Jurim O, Colonna JO, Rosove MH, Busuttil RW. Reversal of neutropenia with granulocyte colony-stimulating factor without precipitating liver allograft rejection. Transplantation 1993; 56: 1593-5. [ Links ]
70. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet 2001; 358: 958-65. [ Links ]
71. Fried MW, Shiffman ML, Reddy KR, Smith C, Marios G, Goncales FL, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975-82. [ Links ]
72. Samuel D, Brousse P. Treatment of patients with recurrent hepatitis C after liver transplantation with pegylated interferon and ribavirin (abstract). Hepatology 2003; 34: 531A. [ Links ]
73. Neumann UP, Langrehr JM, Berg T, Neuhaus P. Treatment of recurrent hepatitis C after liver transplantation with peginterferon alfa-2b and ribavirin (abstract). Hepatology 2003; 34: 531A. [ Links ]
74. Ghobrial RM. Retransplantation for recurrent hepatitis C. Liver Transpl 2002; 8 (Supl. 1): S38-S43. [ Links ]











 texto en
texto en