Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.8 Madrid ago. 2004
| CLINICAL NOTE |
Gastric stromal tumors: clinical presentation and surgical options
C. Pardo Martínez, J. Mayol Martínez, C. Hernández Pérez and J. Álvarez Fernández-Represa
Service of General Surgery I. Hospital Clínico San Carlos. Madrid. Spain
ABSTRACT
Stromal tumors represent 1-3% of all primary gastric neoplasms. These tumors can occur at any age and display different clinical manifestations, but they rarely reach over 10 cm in size. Currently they are designed as gastrointestinal stromal tumors (GIST) but their classification is still controversial. Surgery is the treatment of choice, and the extension of surgical resection depends on the tumor size, neoplastic involvement of adjacent organs, and the presence of metastatic disease. In selected cases minimally invasive surgery can provide excellent results.
We present four new patients with GIST who exemplify the different clinical forms of presentations of GISTs and their diverse treatment options.
Key words: Leiomyoblastoma. Stromal tumors. GIST. Treatment. Surgery.
Pardo Martínez C, Mayol Martínez J, Hernández Pérez C, Álvarez Fernández-Represa J. Gastric stromal tumors: clinical presentation and surgical options. Rev Esp Enferm Dig 2004; 96: 578-583.
Recibido: 28-07-03.
Aceptado: 07-10-03.
Correspondencia: Cristina Pardo Martínez. Servicio de Cirugía I. Hospital Clínico San Carlos. Dr. Martín Lagos, s/n. 28040 Madrid. e-mail: crispardomar@hotmail.com
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are extremely rare, and represent between 1 and 3% of all primary gastric neoplasms (1). These tumors may occur at any age but their peak incidence is during the sixth decade of life. The most frequent localization is the mid part of the stomach, followed by the antrum. In almost 20% of patients the tumor appears near the pylorus, although gastric outlet obstruction secondary to neoplastic growth is uncommon (2). GISTs may vary in size between a few milimeters and 20 cm or more, but only 20% of them may reach a diameter over 10 cm. Up to 30-50% of patients with GISTs are asymptomatic (3,4) at the time of diagnosis, particularly those with small tumors. Since the introduction of CT and ultrasound imaging the difficulties to diagnose these tumors by using non-invasive methods have been greatly overcome (5). Both imaging modalities, together with endoscopic examination with multiple biopsies of the suspected lesion (6) for further immunohistochemical studies, are the most valuable tools for the diagnosis of these neoplasms. They must be used not only for the diagnosis and staging of the primary tumor but also for postoperative follow-up.
Curative surgical resection is the treatment of choice for gastric stromal tumors irrespective of whether they are benign or malignant. However, technical options may vary from a minimally invasive approach to massive gastrointestinal exeresis aimed at achieving disease-free margins.
We report four patients whose clinical scenarios represent the multiple diagnostic and therapeutic options for these neoplasms.
CASE REPORTS
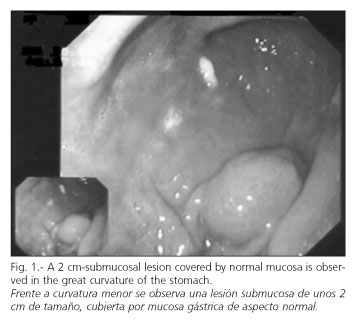
Case 1. A 76-year-old asymptomatic male patient was diagnosed with an extraluminal gastric lump on routine abdominal ultrasound examination. An upper gastrointestinal endoscopy (Fig. 1) showed an extramucosal gastric tumor of 3 cm in size. He underwent an endoscopy-aided laparos-copic gastric wedge resection. Histopathological examination found a neoplastic mesenchymal proliferation with scarce mitoses (fewer than 1/50 high power field). Immunohistochemically, cells were negative for cytokeratin, S-100, desmin and actin, and positive for vimentin. The patient was free of disease at two years and a half after surgery.
Case 2. A 72-year-old female patient presenting with upper gastrointestinal bleeding was diagnosed with a 4 cm ulcerated submucosal tumor located in the gastric greater curvature on upper endoscopic examination. She underwent curative open gastric wedge resection with disease-free margins. The histopathological diagnosis was consistent with a gastrointestinal stromal tumor of undetermined malignant potential. After two years of follow-up extragastric tumor recurrence was found on a CT scan, and she underwent “en-bloc” resection of the antrum, the great omentum and the spleen. No liver metastases were found. Histopathologically, there were peritoneal, omental, gastric and lymph node metastases from a malignant GIST (over 15 mitoses per high power field). It was positive for CD34 and C-KIT (CD117), and negative for muscle markers (actin and desmin) and for S-100, synaptophysin, and Leu-7. The patient is disease-free at 9 months after reoperation.
Case 3. A 71-year-old patient presented at the emergency department with asthenia, weight loss and intermittent abdominal pain for the last 2 months. On the abdominal CT scan a large abdominal tumor of 25x25x13 cm in size with necrotic areas was seen. It extended from the epigastrium downwards to the mesogastrium, thus displacing the stomach but without a defined site of origin. There were no liver implants. The patient underwent elective surgical treatment. An “en-bloc” resection of the stomach and transverse colon was carried out due to the presence of a gastric stromal tumor invading the colon. The histopathological study showed a mesenchymal neoplastic proliferation with 31 mitoses per 50 high power field. Neoplastic cells were negative for cytokeratin, actin, desmin, and S-100, and positive for vimentin. There was no lymphatic involvement. The patient was admitted to the emergency department 8 months after surgery because of severe anorexia and a painful abdominal mass on the upper abdomen. An abdominal CT scan (Fig. 2) showed a local tumor recurrence with lymph node, retroperitoneal, mesenteric, liver and pulmonary metastases. The patient died one month later.
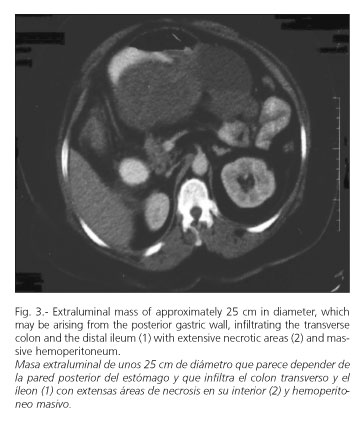
Case 4. A 60-year-old female patient with a previous history of high blood pressure presented to the emergency department with acute abdominal pain accompanied by nausea, vomiting, and hypotension. On physical examination she appeared hypotensive (blood presure 60/40). Her hemoglobin concentration was 8.1 g/dl, and the hematocrit was 24.7%. An abdominal CT scan (Fig. 3) showed a heterogeneous mass (both solid and liquid) of about 20 cm in size emerging from the posterior aspect of the stomach. She underwent emergency surgical treatment for a massive hemoperitoneum and a large necrotic tumor of the stomach invading the distal ileum, the cecum, and both the ascending and transverse colon. Histopathologically, the tumor contained cells organized in bundles with scarce mitoses (fewer that 1/50 high power field) and with a low proliferative index (Ki67). The immunohistochemical study showed that neoplastic cells were negative for actin, desmin, S-100, and keratin, and positive for CD-34 and vimentin. The patient is free of disease at 18 months after surgery.
DISCUSSION
GISTs represent the larger group of non-epithelial neoplasms of the stomach and the small bowel (7-9). The name GIST was first used by Mazur and Clark (10) in 1983, and this category includes an heterogeneous group of tumors with a wide variety of cellular differentiation. This fact has brought some degree of confusion with regard to its interpretation. In the 1980s several immunohistochemical studies showed that these tumors may display extremely variable cellular differentiation (11-14). They may display smooth muscle (as shown by the expression of actin, myosin and desmin), autonomic nerve, ganglionic or neural differentiation. They may also remain poorly differentiated.
In 1998 Kindblom (15) postulated for the first time the possibility that these tumors may derive from the interstitial cells of Cajal, based on the fact that both cell types are positive for the same marker - CD117 (c-KIT).
Several prognostic systems have been proposed, but the one revised in 1993 by UICC, which comprises tumor size, lymph node involvement, presence of distant metastases and immunopathologic examination, is most widely accepted (16). Prognostic factors that are currently accepted include: tumor location (10), tumor size over 5 cm (17), immunohistochemical tumor features (nuclear abnormalities, necrosis, vascular invasion, number of mitoses greater than 5 x 50 high power field, c-KIT mutation) (18,19), involvement of adjacent organs, and presence of metastases (20). Although several authors have found an association between exon 11 mutations in c-kit gene and the more aggressive behavior of these tumors, recently, Bernett et al (21) were not able to confirm this relationship. Some authors also consider tumor rupture during surgery and incomplete surgical resection as risk factors for poor prognosis (22).
The liver is the most frequent site for metastasis, as seen in case 2, followed by the peritoneum and the lung. Metastatic disease may appear as late as 30 years after resection of the primary tumor. Overall, removal of metastatic disease is re-commended because it may increase survival, which is about 50% at 5 years.
Surgery is the treatment of choice for GISTs. No difference in survival at 5 and 10 years (23) has been observed between wide and more limited surgical resection in patients with tumors under 5 cm in diameter and no liver metastases at the time of diagnosis. In these patients, as in case 1, a minimally invasive approach may yield excellent results.
GISTs are resistant to both conventional chemotherapy and radiation (24,25). For patients who have undergone non-curative surgery, imatinib mesylate (Gleevec®) has been recently approved. This drug is in clinical use for the treatment of Philadephia (+) chronic myeloid leukemia. Imatinib mesylate works by blocking the mutated membrane receptor c-KIT, which has intrinsic tyrosine-kinase activity. This mutation is critical for the development of these tumors. By blocking this receptor, the drug blocks tumor proliferation (26) and induces cell apoptosis (27).
Recent studies (28) have shown that a subgroup of GISTs lacks the c-KIT mutation but displays a mutation of the platelet-derived growth factor receptor alpha (PDGFRA), which possesses intrinsic tyrosine-kinase activity. This mutation is identified by immunoprecipitation with polyclonal antibodies against polypeptides with specific sequences of the tyrosine-kinase receptor family. Both mutations, c-KIT and PDGFRA, appear to be alternative and mutually exclusive. Therefore, the absence of a c-KIT mutation does not exclude the diagnosis of GIST and warrants further study of a PDGFRA mutation. If a stromal tumor does not express the c-KIT mutation, Gleevec® (28) may still be considered a therapeutic option. In both preclinical (29) and clinical studies (30) imatinib mesylate has been shown to be effect-ive for patients with unresectable or metastatic disease, thus representing the only available therapeutic modality for these patients.
REFERENCES
1. Cocco P, Ottomello R, Gemini S, Maxia G, Cdoni S. Il leiomioblastoma gástrico. Pressentazione di un caso. Minerva Chir 1992; 47: 1727-30. [ Links ]
2. Delgado Millán MA, Abad Barahona A, Morales Gutiérrez C, Martín Díaz M, López López A, Sierra García A, et al. Leiomiosarcoma gástrico, a propósito de un caso. Rev Esp Enferm Dig 1991; 80: 213. [ Links ]
3. Palmiere B, Cogni P, Criscuolo M, Di Gregorio C. Leiomioblastoma dello stomaco: Ulteriore contributo casistico. Minerva Chir 1991; 46: 561-6. [ Links ]
4. Sacco D, Paron L, Bardella R, Spidalieri G, Cardino L. Leiomiosarcoma gástrico. Osservazione di tre casi clinici in urgenza. Minerva Chir 1994; 49: 995-9. [ Links ]
5. Poza P, Pombo P, de Juan Rumero P, Gómez-Chao de Santos T, Montero Gómez M. Hipoglucemia asociada a un leiomiosarcoma gástrico. Rev Esp Enferm Dig 1996; 88: 359-60. [ Links ]
6. Zullo A, Botto G, Demaria G. Leiomioblastoma gastrico epitelioide. Descrizione di un caso clinico e considerazioni. Minerva Chir 1994; 49: 207-10. [ Links ]
7. Appelman HD, Helwig EB. Gastric epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma). Cancer 1976: 709-28. [ Links ]
8. Manconi A. Su un caso de leiomioblastoma dello stomaco. Minerva Med. 1990; 81 (Supl. 7-8): 71-4. [ Links ]
9. Cattaneo U, Andreone D, Enrico S, Serra GC, Toppino M, Corno F, et al. Il rabdomiosarcoma: presentazione di un caso a localizzazione addominale. Minerva Chir 1991; 46: 417-20. [ Links ]
10. Crosby JA, Catton CN, Davis A, et al. Malignant Gastrointestinal Stromal Tumors of the Small Intestine: A review of 50 cases fron a prospective database. Annals of Surgical Oncology 2001; 8: 50-9. [ Links ]
11. Rosay J. Gastrointestubak tract: stromal tumors. En: Ackerman'. Surgical Pathology. 8ª ed. Mosby, 1996. p. 645-7. [ Links ]
12. Miettinen M. Gastrointestinal stromal tumors. An immunohistochemical study of cellular differentiation. Am J Clin Pathol 1988 (89): 601-10. [ Links ]
13. Franquemont DW. Diferentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol 1995; 103: 41-7. [ Links ]
14. Suster S. Gastrointestinal stromal tumors. Semin Diagn Pathol 1996; 13: 297-313. [ Links ]
15. Kindblom LG, Remotti HE, Aldemborg F: Gastrointestinal pacemaker cell tumor (GIPACT). Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998; 152: 1259-69. [ Links ]
16. Hermanek P, Henson DE, Hutter RVP, Sobin LH. IUCC TNM Suplement 1993: A commentary on Uniform Use. Berlin, 1993: Springer. Verlag. [ Links ]
17. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brenn MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231: 51-8. [ Links ]
18. Yan H, Marchettini P, Acherman YI, Gething SA, Brun E, Sugarba PH. Prognostic assessment of gastrointestinal stromal tumor. Am J Clin Oncol 2003; 26: 221-8. [ Links ]
19. Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002 15; 20: 3898-905. [ Links ]
20. Shiu MH, Farr GH, Papachristou DN, Hadju SI. Myosarcomas of the stomach. Natural history, prognostic factors and management. Cancer 1982 (49): 177-87. [ Links ]
21. Bernet L, Bustamante M, Zúñiga A, Cano R. Characterization of GIST/GIPACT tumors by immunohistochemistry and exon 11 analysis of c-kit gene by PCR. Rev Esp Enferm Dig 2003; 95: 688-91. [ Links ]
22. Ng EH, Pllock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992: 69: 68-77. [ Links ]
23. Cabello Rodríguez M, Rueda Pérez JM, Somaza de Saint-Palais M, Fernández Lobato R. Leiomioblastoma. Tumor de histogénesis controvertida. Rev Esp Enferm Dig 1991; 80: 352-3. [ Links ]
24. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000 (231). 51-8. [ Links ]
25. Goss GA, Merriam P, Manola J, Singer S. Fletcher CD, Demetri GD. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol 2000; (19): 599-abstract. [ Links ]
26. Solís-Herruzo JA, Solís-Muñoz P. GISTs: from the molecular knowledge to the racional treatment. Rev Esp Enferm Dig 2003; 95: 677-82. [ Links ]
27. Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Oh KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI-571, a selective tyrosine kinase inhibitor. Blood 2000; 96: 925-32. [ Links ]
28. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CL, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003; 299 (5607): 708-10. [ Links ]
29. Tuveson DA, Willis NA, Jacks T, et al. STI-571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene 2001; 20: 5054-8. [ Links ]
30. Demetri GD, Von Mehren M, Blake CD, Van den Abbeele AD, et al. Efficacy and safety of Imatinib Mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472-80. [ Links ]











 texto en
texto en