Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.12 Madrid dic. 2004
| ORIGINAL PAPERS |
Post-transfusional hepatitis in neonates hospitalized in a Neonatal Intensive Care Unit
A. Ruiz-Extremera, J. Salmerón1, M. L. Rey, C. Torres1, P. Muñoz de Rueda1, E. Ocete and J. D. Luna2
Department of Pediatrics. 1Department of Gastroenterology. San Cecilio University Hospital. 2Department of Biostatistics.
School of Medicine. University of Granada. Granada. Spain
ABSTRACT
Objective: to assess the significance of increased serum transaminase levels in neonates admitted to a Neonatal Intensive Care Unit and its relationship with blood transfusion.
Methods: follow-up prospective study of 209 patients; 177 completed follow-up, of whom 129 were transfused and 48 were not; 57 were born after full gestation and 120 were born prematurely. The activity of serum levels of ALT, AST, and GGT was measured monthly up to six months of age, and until six months after the last transfusion. At the end of follow-up, and whenever an increase in serum transaminase levels was detected, the viral agents of hepatitis A, B, C, G, TT, cytomegalovirus, Epstein-Barr, and herpes 1 and 2, and toxoplasma were studied. Viral serology was also carried out in mothers and in donors when children tested positive.
Results: one hundred twenty nine neonates (73%) received 461 U red blood cell transfusions (3.6 ± 3 U/patient). ALT levels increased in 54 (30.5%) patients, of whom 46 (36%) were transfused and eight (17%) were not (p < 0.05). The independent variables were ‘infection by G virus' and ‘parenteral nutrition for more than 12 days'; the variable ‘transfusion' was close to the limit for statistical significance. Twenty patients (11.3%) had increased serum ALT levels 2.5 times above the normal value: 18 (14%) were transfused and two (4%) were not (p = 0.106). Only the G and TT viruses were related with transfusion; patients remained asymptomatic, although most neonates were chronically infected.
Conclusion: follow-up showed that increased serum ALT levels are common among severely ill neonates. Blood transfusions are safe concerning most hepatotropic viruses, but transmission of viruses G and TT is possible.
Key words: Post-transfusional hepatitis. Newborn babies. Premature infant. Neonatal Intensive Care Unit (NICU). Hepatitis G virus. Hepatitis TT virus.
Ruiz-Extremera A, Salmerón J, Rey ML, Torres C, Muñoz de Rueda P, Ocete E, Luna JD. Post-transfusional hepatitis in neonates hospitalized in a Neonatal Intensive Care Unit. Rev Esp Enferm Dig 2003; 96: 835-846.
Financial support: Health Research Fund FIS 95/1524, Junta de Andalucía Nº 84/99, and National Network of Hepatology and Gastoenterology Research (RNIHG)
Recibido: 29-01-04.
Aceptado: 04-05-04.
Correspondencia: Ángeles Ruiz-Extremera. Departamento de Medicina Pediátrica. Hospital Universitario de San Cecilio. Avda. Dr. Olóriz, 16. 18012 Granada. Tel.: 958 023 411. Fax: 958 240 740. e-mail: arextrem@ugr.es.
INTRODUCTION
Therapy for critically ill patients typically includes multiple red blood cell (RBC) transfusions (1). Newborn babies (NBs) admitted to Neonatal Intensive Care Units (NICU) are commonly given transfusions of RBC. In the USA it has been estimated that 80% of NBs weighing less than 1.500 g are transfused (2,3). In recent years, guides to clinical practice have been published to standardize criteria for transfusion. Although the administration of human alpha-recombinant erythropoietin (EPO) has allowed decreasing the rate of transfusions, this reduction seems also related to stricter transfusion criteria (2-4). Common indications for neonatal RBC transfusions include volume expansion, cardiorespiratory disease, anemia, growth failure, and apnea. Also used are predictors such as demographic criteria, estimated phlebotomy blood loss, initial hematocrit (3,4), and birth weight (5).
Among NBs weighing less than 1.000 g, an early use of EPO does not reduce the number of transfusions or the number of NBs requiring them (6). It has also been reported (7) that apparently stable anemic NBs show echocardiographic evidence of hemodynamic changes that do not improve after 48 h; this situation is undesirable and potentially dangerous. Another noteworthy fact is the variability of clinical practice among NICUs (8). Although several publications have attempted to clarify indications, the efficacy and safety of clinical practice remains ill-defined (9).
Published studies of post-transfusional hepatitis (PTH) among children are scarce and retrospective (10,11), but studies of reduced PTH have been carried out among adults. Hepatitis C virus (HCV) was found to be the main cause of non-A non-B PTH. However, since anti-HCV testing of blood donors was established as mandatory, PTH has decreased to 0.85 to 3.5% (12-14), and the possibility of transmitting an infection by HCV is practically nil (15).
Recently, new parenterally transmitted viral agents have been recognized, namely hepatitis G virus (HGV) and transfusion-transmitted virus (TTV). HGV was detected in 8.6% of a group of transfused adults, although none of them met criteria for PTH (15), and no hepatic replication of this virus has been shown (16). TTV is transmissible from mother to child through the blood (17), and also via a fecal-oral route (18). It has been detected in patients with non-A to non-G PTH (19).
The objective of the present study was to determine the significance of increased serum transaminase levels among NBs admitted to a NICU, as well as the safety of the transfusions were performed.
METHODS
From May 1995 to December 1998, a prospective follow-up study was carried out on 209 NBs with previous informed parental consent and the approval of the Hospital Ethics Committee; 177 NBs completed the study, of whom 129 were transfused and 48 were not. Thirty-two were excluded because of loss to follow-up. Of the patients that completed the study, 57 were born at full term and 120 were born prematurely. Fifty-six of the NBs weighed less than 1.500 g at birth. All were admitted to a NICU and subsequently discharged from hospital.
The follow-up was carried out monthly until the age of six months or until six months after the last transfusion. For the children who presented increased serum ALT levels, follow-up was prolonged with half-yearly visits. The mean follow-up period was 18.7 ± 9 months (range, 12 to 48 months). At each check-up serum levels of ALT, AST, and GGT were measured, and at 3 and 6 months, 1 mL of serum was frozen at -80 °C. During the last check-up, and whenever an increase in ALT was observed, the following viral agents were studied: hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes virus (HV 1 and 2), HGV, and TTV. Due to the possibility of vertical transmission, a virologic study was performed in all mothers and blood donors, and serum samples were frozen.
HGV and TTV were only investigated in mothers if their children tested positive. All units of the packed cells transfused were negative for anti-HCV (ELISA 3), HBsAg, HIV, and syphilis. Donors with increased levels of ALT were rejected. The classical criteria for PTH were applied, namely an increase in serum levels of ALT 2.5 times above normal during a period of 14-180 days post-transfusion, confirmed by a second test (20).
Biochemical parameters were measured using a HITACHI-717 (Boehringer-Mannheim) automatic analyzer. The markers for HAV (anti-HAV IgM), HBV (anti-HBc IgM and HBsAg), and CMV were studied by ELISA. EBV was determined by indirect immunofluorescence. HCV was tested for anti-HCV by ELISA 3, RIBA3 (Ortho). HCV-RNA was determined in the serum by qualitative RT-PCR using the Amplicor HCV Kit (Roche Diagnostics System) according to the manufacturer's instructions.
The analysis of HGV-RNA was carried out by means of amplification and detection of two independent genomic regions: the 5' non-coding region and the region coding for the NS5a protein. The detection of these two regions was carried out in independent RT-PCR reactions (Boehringer-Mannheim). This method was employed singly for patients with increased levels of ALT, and in pools to study patients with normal levels of transaminases; positive tests were repeated. For the study of antibodies, we used an ELISA (Boehringer-Mannheim) technique, which qualitatively detected anti-HGVE2.
TTV-DNA was extracted from 150 mL of serum using the Nucleo Spin Virus Kit (Clontech Laboratories GmbH) and resuspended in 50 µL of buffered solution. TTV-DNA was amplified by semi-nested PCR with TTV-specific primers derived from two regions preserved from published sequences (21). For the first PCR, the primers TT6 (sense: 5'ACAGACAGAGGAGAAGGCAA) and TT7 (antisense: 5'TAC CAYTTAGCTCTCATT, with Y=C and T) (22) were used to amplify a product of 329 bp. After electrophoresis on agarose gel, samples producing a single band of 267 bp (the expected size for the amplified TTV-DNA fragment) were considered positive.
Statistical method
For qualitative variables, the statistical χ2 or two-tailed exact Fisher's test was used, and the odds ratio (Ô) and confidence intervals (CI) were estimated. For the analysis of quantitative variables, a Wilcoxon's test for independent samples was carried out. In all tests, a statistical significance level of 95% was taken. Exact logistic regression analysis (Logxact) was used to assess the independent effects of significant variables in univariate analysis. The final models were built in a three-step manner: in the first one, individual effects were considered (model 0); in the second step, the whole set of variables was used (model I), and in the third step (model II), only significant variables in the previous model were considered.
RESULTS
The gestation period was 34.4 ± 4.5 weeks (24-43 weeks), and the weight at birth was 2195 ± 944 g (range, 550-4350 g). One hundred fifty nine 159 NBs (89%) were treated with antibiotics, 109 (62%) received machine-assisted ventilation lasting an average of 13 ± 15 days (1-80 days), 82 (46%) were treated with vasoactive drugs, and 74 (42%) received parenteral nutrition for an average of 25 ± 20 days (2-99 days). Mean stay at the NICU was 28 ± 28 days (range, 2-180 days), and total hospitalization period was 38.5 ± 31 days (range, 5-195 days). Of the 177 NBs, 129 (73%) needed transfusion and received 461 units (U), with an average of 3.6 ± 3 U (1-21 U per patient).
Patients who had increased serum ALT levels
ALT levels were higher in 54 (30.5%) patients, 46 (36%) of whom were transfused, and eight (17%) were not transfused (p < 0.05). Table I and II show the results of the univariate and multivariate analysis, respectively.
Patients who had serum ALT levels 2.5 times above normal values
Only 20 (11.3%) children had ALT 2.5 times above the normal value; 14% of them were transfused and 4% were not (p =0.106). Table III shows the results of the univariate analysis, and table IV those of the multivariate analysis.
Patients who had an increase in serum AST and GGT levels
In all, 128 patients (72%) had an increase in serum AST levels (81.7 ± 128 U/L; 95% CI 59-104 U/L). Of these, 93 (73%) were transfused and 36 (73%) were not.
Serum GGT levels were increased in 64 (36%) patients (136 ± 146 U/L; 95% CI 95-176 U/L). This increase correlated with a shorter gestation period (p < 0.01), a low weight at birth (p < 0.05), an increased serum ALT levels (p < 0.01), PTH (p < 0.05), and, at the limit of statistical significance, with transfusion (p = 0.065) and parental nutrition (p = 0.096).
Viral study
We did not observe any patient with HAV, HBV, HCV, HV or EBV infection. One child who was not transfused had infection by CMV at 5 months of age, with serum ALT levels of 75 U/L that persisted for one month. A study of the mother proved negative for CMV.
HGV was detected in 15/177 (9%) neonates; 14 were HGV-RNA-positive and one was considered to be infected in spite of being HGV-RNA-negative, since seroconversion to anti-HGV E2 antibody occurred during the course of follow-up. Of these neonates, 11/129 (9%) had been transfused and 4/48 (8%) had not. Differences were not statistically significant.
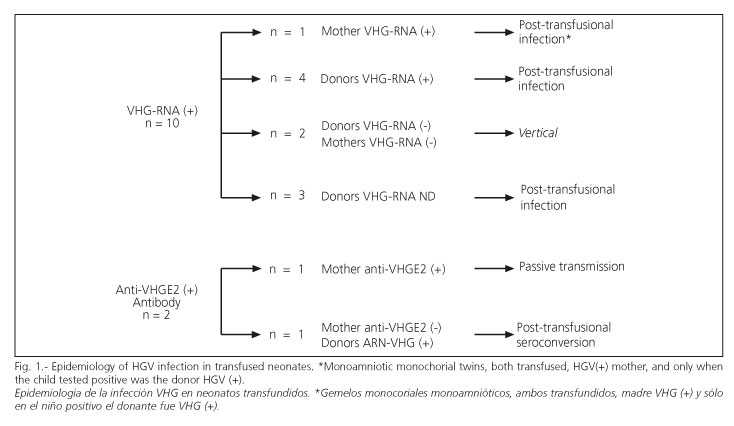
Figure 1 shows epidemiological features of HGV infection among transfused neonates, who had nine cases of parenteral infection and two of vertical infection. The rate of HGV infection by transfusion was 7% (95% CI, 3-13%). Patients with post-transfusion HGV infection received an average of 6±5 U (range, 1-14 U) concentrated RBC. The transfused and HGV-negative patients received 3 ± 3 U (range, 1-22 U). Difference between means was not statistically significant. The risk of HGV transmission per transfused unit of RBC was 1.96% (95% CI, 1-3.8%). The mothers of the four non-transfused HGV-RNA-positive NBs were also HGV-RNA-positive (vertical transmission).
An anti-HGV E2 study was carried out in all NBs, and was found to be negative in all of them, except in one who was positive at 6 months and negative three months later. The mother of this NB showed anti-HGV E2 antibody positive, therefore it was considered that the positive antibody in this child resulted from a passive transmission.
The clinical course of the HGV infection was mild (Fig. 2). Fourteen NBs (93%) presented increased ALT, but only in five (33%), serum ALT levels were higher than 2.5-fold above the normal value. The maximum ALT level recorded was 78 ± 46 U/L, and the mean duration was 3 ± 2 months. Only four patients were virus-free at 18, 36, and 48 months, and 1 neonate sero-converted to anti-HGV E2 antibody. The remaining 11 patients remained HGV-RNA-positive, but ALT was normal.
TTV-DNA was studied in 127 neonates, of whom was positive in 39 (31%). Of these, 36/109 (33%) had been transfused and 3/16 (17%) had not been transfused. This difference was not statistically significant. In the transfused group, there were five cases of vertical transmission, since mothers were also positive. The remaining mothers tested negative and parenteral transmission was considered most likely, although serum from the donors was not available. The mothers of three cases in the non-transfused group were TTV-DNA-positive; therefore, a vertical transmission was most likely. In seven cases, there was a co-infection by HGV.
The clinical course of the TTV infection was asymptomatic. Sixteen neonates showed increased serum ALT levels, and six of them were co-infected with the HGV. Of these, eight (50%) had an increase in serum ALT levels 2.5 times above the normal value; three of these were co-infected with the HGV. The maximum level recorded for ALT was 87 ± 81 U/L, and the mean duration was 4±3 months. Only two children had cleared up the virus at 6 and 18 months, the others children remaining positive, although with normal serum ALT levels.
Serologic study of mothers
Serologic studies carried out in mothers showed that 76 (47%) had anti-CMV IgG antibody, 46 (28%) anti-HAV IgG, 39 (25%) anti-EBV IgG, 27 (17%) anti-HVs IgG, 11 (7%) anti-toxoplasmca IgG, 7 (4%) anti-HBs, 2 (1%) anti-EBV IgM and one (0.6%) anti-CMV IgM antibodies. In the latter two cases, no repercussions on the children were observed.
Discussion
To date, the real prevalence of post-transfusional pathology among premature and full-term neonates remains unknown. For this reason, we analyzed factors related with increased levels in serum ALT levels in these patients. Patients who were most severely ill and those with the lowest weight at birth required a longer period of hospitalization in the NICU, although not all of them underwent a higher number of therapeutic procedures, including transfusion.
Seventy percent of neonates admitted to the NICU had no increase in serum ALT levels. Neither weight nor gestational age were related with increased serum levels of ALT. Transfusion, number of units transfused, duration of hospitalization period in the NICU, duration of mechanically-assisted ventilation, and duration of parenteral nutrition were all related with increased ALT levels. Most of the severely ill neonates received all these interventions; differences between them concerned the duration of such interventions; thus, variables are interrelated each other. In contrast, HGV infection, duration of parenteral nutrition, and the fact of having received transfusions are independent variables within the logistic regression analysis. Póstuma, et al. (23) described a transitory increase in serum levels of alkaline phosphatase and ALT in 34% of NBs after 4-6 weeks of parenteral nutrition. Other authors have reported the presence of liver complications in low-weight, premature NBs (24-26). In our study, increased serum ALT levels were not related with weight or gestation period.
Hepatitis among adults has been widely studied, and it is known that it represents a risk factor of post-transfusional disease. In the present study, 14% of the transfused neonates, and 4% of the non-transfused neonates presented an increase in the serum ALT levels 2.5 times above the normal value. HGV was responsible for only five cases, and in three of them, a TTV co-infection was also present. This means that increased serum ALT levels results from other factors, which may be related with the main illness that led to the admission into the NICU or to other unidentified transfusion-transmitted viruses (27). Increased serum levels of AST was also common, although less specific. Note the number of patients who presented increased serum GGT levels; this was more frequent among low-birth-weight neonates, and it is likely related to a certain degree of cholestasis.
A surprising finding is the high prevalence of the TTV infection (31%). In Spain, the prevalence of this infection among healthy blood donors is 14 and 76% among patients with hemophilia (28). Vertical transmission of HGV and TTV is important and responsible for infection in non-transfused and some transfused neonates. Accordingly, it is essential to study mothers and blood donors in order to interpret the importance of transfusion as the factor responsible for transmission of these viruses. Some authors have reported the vertical transmission of HGV and TTV infections (29,30) and appears to be more frequent than that of HCV infection (31).
In conclusion, this study shows how important it is to monitor levels of serum ALT levels in NBs hospitalized in NICUs, as such patients are already subject to multiple factors that may give rise to liver disease. We should also underline the importance of virologic studies, not only of NBs but also of mothers and blood donors. Although HGV has not been clearly related with liver lesions in adults, in NBs it may cause increased levels of serum levels of ALT. Finally, it should be underlined that transfusions are safe regarding most viruses, but the transmission of new agents such as HGV and VTT, whose pathogenicity is very low, is possible.
REFERENCES
1. Corwin HL, Gettinger A, Pearl RG, Fink MP, Levi MM, Shapiro MJ, et al. Efficacy of recombinant human erythropoietin in critically ill patients: A randomized controlled trial. JAMA 2002; 288: 2827-35. [ Links ]
2. Strauss RG. Erythropoietin and neonatal anemia. N Engl J Med 1994; 330: 1227-8. [ Links ]
3. Shannon KM, Keith JF, Mentzer WC, Ehrenkranz RA, Brown MS, Widness JA, et al. Recombinant human erithropoietin stimulates erythropoiesis and reduces erythrcyte transfusions in very low birth weight preterm infants. Pediatrics 1995; 95: 1-10. [ Links ]
4. Strauss RG. Practical issues in neonatal transfusion practice. AJCP 1997; 107 (4) (Supl. 1): S57-63. [ Links ]
5. Maier RF, Obladen M, Messinger D, Wardrop C. Factors related to transfusion in very low birthweight infants treated with erythropoietin. Arch Dis Child Fetal Neonatal Ed 1996; 74: F182-6. [ Links ]
6. Ohls RK, Ehrenkranz RA, Wright LL, Lemons JA, Korones SB, Stoll BJ, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: A multicenter, randomized, controlled trial. Pediatrics 2001; 108: 934-42. [ Links ]
7. Alkalay AL, Galvis S, Ferry DA, Simmons CF, Krueger RC. Hemodynamic changes in anemic premature infants: Are we allowing the hematocrits to fall too low? Pediatrics 2003; 112: 838-45. [ Links ]
8. Ringer SA, Richardson DK, Sacher RA, Keszler M, Churchill WH. Variations in transfusion practice in Neonatal Intensive Care. Pediatrics 1998; 101: 194-200. [ Links ]
9. Editorials. Practice variation: Implications for neonatal red blood cell transfusions. J Pediatr 1998; 133: 589-90. [ Links ]
10. Blajchman MA, Sheridan D, Rawls WE. Risks associated with blood transfusion in newborn infants. Clin Perinatol 1984; 2: 403-15. [ Links ]
11. Keller KM, Wirth S. Hepatitis C virus (HCV). Low infection rate in premature and full-term neonates following multiple transfusions. Monatsschr Kinderheilkd 1992; 140 (2): 108-12. [ Links ]
12. González A, Esteban JI, Madoz P, Viladomiu L, Genesca J, Muñiz E, et al. Efficacy of screening donors for antibodies to the hepatitis C virus to prevent transfusion-associated hepatitis: final report of a prospective trial. Hepatology 1995; 22: 439-45. [ Links ]
13. Salmerón J, Gila A, Oyonarte S, Palacios A, Pérez Ruiz M, Fernández Montoya A, et al. Estudio prospectivo de la incidencia de hepatitis postranfusional después de la exclusión de los donantes anti-VHC ELISA 2 positivos. Med Clin 1995; 105: 641-4. [ Links ]
14. Barrera JM, Bruguera M, González M, Gálvez A, Castillo R, Rodés J. Eficacia de la exclusión de donantes anti-VHC positivo por ELISA 2 en la prevención de la hepatitis postransfusional. Gastroenterol Hepatol 1996; 19: 240-2. [ Links ]
15. Salmerón J, Carmona I, Torres C, Muñoz de Rueda P, Gila A, Ruiz-Extremera A. Estudio retrospectivo de la incidencia de infección por el virus de la hepatitis G en los pacientes transfundidos. Med Clin 1999; 112: 409-11. [ Links ]
16. Torres C, Muñoz de Rueda P, Ruiz-Extremera A, Quintero D, Palacios A Salmerón J. Genomic and antigenomic chains of hepatitis C virus and hepatitis G virus in serum, liver and peripheral blood mononuclear cells. Rev Esp Enferm Dig 2002; 94: 659-63. [ Links ]
17. Sugiyama K, Goto K, Ando T, Mizutani F, Terabe K, Kawabe Y, et al. Route of TT Virus infection in children. J Med Virol 1999; 59: 204-7. [ Links ]
18. Okamoto H, Akahane Y, Ukita M, Fukuda M, Tsuda F, Miyakawa Y, et al. Fecal excretion of a nonenveloped DNA virus (TTV) associated with posttransfusion non-A-G hepatitis. J Med Virol 1998; 56 (2): 128-32. [ Links ]
19. Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, MiyaKawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun 1997; 241: 92-7. [ Links ]
20. Esteban JI, González A, Hernández JM, Viladomiu L, Sánchez C, López-Talavera JC, et al. Evaluation of antibodies to hepatitis C virus in a study of transfusion associated hepatitis. N Engl J Med 1990; 323: 1107-12. [ Links ]
21. Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Lizuka H, et al. Molecular cloning and characterization of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatology Research 1998; 10: 1-16. [ Links ]
22. Höhne M, Berg T, Müller AR, Schreier E. Detection of sequeences of TT virus, a novel DNA virus, in german patients. J General Virology 1998; 79: 1-4. [ Links ]
23. Postuma R, Trevenen CL. Liver disease in infants receiving total parenteral nutrition. Pediatrics 1979; 63: 110-5. [ Links ]
24. Bell Rl, Ferry GD, Smith EO, Shulman RJ, Christensen BL, Labarthe DR, Wills CA. Total parenteral nutricion-related cholestasis in infants. JPEN 1988; 2: 602-6. [ Links ]
25. Howell RR. The diagnostic value of serum enzyme measurements. J Pediatr 1996; 68: 121-34. [ Links ]
26. Brown MR, Thunberg BJ, Goloub L, Maniscalo WM, Cox C, Shapiro DL. Decresed cholestasis with enteral instead of intravenous protein in the very low-birth weight infants. J Pediatr Gastroenterol Nutr 1989; 9: 21-7. [ Links ]
27. Tanaka Y, Primi E, Wang RY, Umemura T, Yeo AE, Mizokami M, et al. Genomid and molecular evolutionary analysis of a newly identified infectious agent (SEN virus) and its relationship to the TT virus family. J Infect Dis 2001; 183: 359-67. [ Links ]
28. Puig-Basagoiti F, Cabana M, Guilera M, Giménez-Barcons M, Sirera G, Tural C, et al. Prevalence and route of transmission of infection with a novel DNA virus (TTV), hepatitis C virus, and hepatitis G virus in patients infected with HIV. J Acquir Immune Defic Syndr 2000; 23 (1): 89-94. [ Links ]
29. Feucht HH, Zölner B, Polywka S, Laufs R. Vertical transmision of hepatitis G. Lancet 1996; 347: 615-6. [ Links ]
30. Davidson F, MacDonald D, Mokili JL, Prescott LE, Graham S, Simmonds P, et al. Early acquisition of TT virus (TTV) in an area endemic for TTV infection. J Infect Dis 1999; 179 (5): 1070-6. [ Links ]
31. Ruiz-Extremera A, Salmerón J, Torres C, De Rueda PM, Giménez F, Robles C, et al. Follow-up of transmission of hepatitis C to babies of human immunodeficiency virus-negative women: the role of breast-feeding in transmission. Pediatr Infect Dis J 2000; 19 (6): 511-6. [ Links ]











 texto en
texto en