Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.12 Madrid dic. 2004
| ORIGINAL PAPERS |
Endoscopic ultrasound-assisted endoscopic resection of carcinoid tumors of the gastrointestinal tract
D. Martínez-Ares, J. Souto-Ruzo, M. J. Varas Lorenzo1, J. C. Espinós Pérez1, J. Yáñez López, R. Abad Belando1, P. A. Alonso Aguirre, J. M. Miquel Colell1 and J. L. Vázquez Iglesias
Service of Digestive Diseases. Complejo Hospitalario Universitario Juan Canalejo. A Coruña. 1Centro Médico Teknon. Barcelona. Spain
ABSTRACT
Introduction: usually found in the gastrointestinal tract, carcinoids are the most frequent neuroendocrine tumors. Most of these lesions are located in areas that are difficult to access using conventional endoscopy (small intestine and appendix); carcinoid tumors found in the gastroduodenal tract and in the large intestine can be studied endoscopically; in these cases, if localized disease is confirmed, local treatment by endoscopic resection may be the treatment of choice. Since endoscopic ultrasonography has been shown to be the technique of choice for the study of tumors exhibiting submucosal growth, the selection of patients who are candidates for a safe and effective local resection should be based on this technique.
Patients and method: we selected patients with gastrointestinal carcinoid tumors who were endoscopically treated between 1997 and 2002. Those patients with tumors measuring less than 10 mm, which had not penetrated the muscularis propria, and those with localized disease were considered candidates for endoscopic resection. The endpoints of this study were to assess the effectiveness (complete resection) and safety (complications) of the technique. Follow-up consisted of eschar biopsies performed one month and twelve months after the resection.
Results: during the aforementioned period, we resected endoscopically 24 tumors in 21 patients (mean age: 51.7 years; 71.5% males). Most lesions were incidental discoveries made during examinations indicated for other reasons. Resection was indicated in most cases as a result of the suspected presence of a carcinoid tumor after endoscopic ultrasonography. Endoscopic ultrasonography also enabled us to clearly identify the layer where the lesion had originated, as well as the size of the lesion. The carcinoid tumor was removed in 13 cases (54.2%) by using the conventional snare polypectomy technique, in 9 cases (37.5%) assisted by a submucosal injection of saline solution and/or adrenaline, and in 2 cases (8.3%) after ligating the lesion with elastic bands. In all cases the resection was complete, with no recurrence during the follow-up period, and no major complications, except for a single case in which a post-polypectomy hemorrhage occurred that was endoscopically solved.
Conclusions: in properly selected patients, the endoscopic resection of carcinoid tumors is a safe and effective technique that permits a complete resection in all cases with few complications. Endoscopic ultrasonography is the technique of choice for selecting the patients who are candidates for endoscopic resection.
Key words: Carcinoid tumors. Management. Endoscopic resection. Complications. Endoscopic ultrasonography.
Martínez-Ares D, Souto-Ruzo J, Varas Lorenzo MJ, Espinós Pérez JC, Yáñez López J, Abad Belando R, Alonso Aguirre PA, Miquel Colell JM, Vázquez Iglesias JL. Endoscopic ultrasound-assisted endoscopic resection of carcinoid tumors of the gastrointestinal tract. Rev Esp Enferm Dig 2004; 96: 847-855.
Recibido: 23-03-04.
Aceptado: 11-05-04.
Correspondencia: David Martínez Ares. C/ Alcalde Gregorio Espino, 77, 5º B. 36205 Vigo. Pontevedra. Telf.: 981 128 914. e-mail: martinezares7@hotmail.com
INTRODUCTION
Carcinoid is the most frequent neuroendocrine tumor (1), with an annual incidence of 2-2.4 cases per 100,000 inhabitants; 74% of these tumors arise in the digestive tract (2); their most common locations are the small intestine and the appendix (1,2), having the latter the best prognosis (2).
Only carcinoids located in the gastroduodenal area (3% of total) as well as those located in the colon and rectum (10 and 11%, respectively) are accessible to endoscopic study (2). Local resection is only considered in cases of localized disease. Thus, type 1 and 2 gastric carcinoids less than 10 mm in diameter (fewer than 2% are metastatic) can be endoscopically removed. On the other hand, tumors located in the colon, usually in the right colon and slightly symptomatic, are diagnosed in more advanced phases (3). In the rectum, up to 85% are found when diagnostic tests are performed for other reasons, and therefore are incidental findings. Like gastric carcinoids, fewer than 2% of rectal tumors with a diameter below 1 cm are metastatic, in contrast with 60-80% of those measuring more than 2 cm (4,5). In addition, when endoscopic ultrasound (EUS) shows that the muscular layer remains unscathed (4,6), local treatment can be safely and effectively applied. When the size of the tumor ranges from 1 to 2 cm, the decision regarding what therapeutic attitude to adopt can be difficult. It is estimated that 10-15% of tumors have metastases (7), which imply that the treatment of choice will depend on the patient's surgical risk. Only in patients with unassumable risk will local treatment be applied (8).
Carcinoids are submucosal tumors, with growth in any of the layers of the wall of the gastrointestinal tract underneath a tumor-free mucosa (9). They originate in cells of the gastrointestinal stroma (10). Most carcinoids are discovered incidentally during examinations indicated for other reasons (11). Conventional endoscopy is greatly limited in the diagnosis of submucosal tumors, as endoscopic biopsies are rarely conclusive (9,11,12). Furthermore, cytology samples taken from these lesions by fine-needle aspiration (FNA) are not very profitable (11,13-16). In addition, the diagnosis of malignancy is based on a series of parameters that, in many cases, can only be studied on examining resected tissue (17).
Endoscopic resection of submucosal tumors has been shown to be a safe, effective technique (18-22) that is clearly superior to endoscopic biopsy in these cases (23). Since removal is the most appropriate way to reach a diagnosis in this type of lesion, tumors will have to be removed when-ever the lesion reaches a large size, shows significant growth, becomes complicated by obstructive symptoms or bleeding, and if diagnostic doubts exist (11,24).
EUS is the technique of choice for the study of these lesions conducted prior to resection (11,25,26). In fact, it enables the physician to precisely differentiate submucosal tumors from extrinsic compressions (11,19,29-31), and solid masses from cystic lesions (9); moreover, it evaluates the layer where the tumor originated with a high degree of precision (24,32), as well as the tumor's size (27). In addition, it may preoperatively establish the type of lesion involved (11,24,34-37), and has proven highly accuracy in predicting malignant behavior (26,28,29,38).
In this study we will discuss our results in the EUS-assisted endoscopic resection of carcinoid tumors. The objective of this paper is to show how EUS makes it possible to select patients that will benefit from local treatment, since a correct evaluation of the size and tumor-originating tissue layer ensures a complete and safe resection in most cases.
PATIENTS AND METHOD
Patient selection
Patients having undergone endoscopic resection for gastrointestinal carcinoids between January 1st, 1997 and December 31st, 2002 at "Juan Canalejo" Hospital, A Coruña, and "Centro Médico Teknon", Barcelona, Spain, were selected. Patients were subsequently subjected to prospective follow-up, which included only those who were subject to a minimum follow-up of 12 months.
Methodology
All patients in whom a digestive submucosal lesion was identified underwent an echoendoscopic study using an Olympus GF-UM 160 radial scanning echogastrofiberscope and an Olympus CF-UM 20 echocolonoscope. When this examination defined a lesion considered suitable for resection, an endoscopic resection was performed using the Olympus GIF-100 and Olympus GIF 120 gastroscopes, or an Olympus GIF 100 colonoscope. The resection was accomplished with a polypectomy snare by applying three different techniques: the conventional snare polypectomy technique, a resection assisted by the submucosal injection of physiological saline solution and/or diluted adrenaline, or a resection assisted by ligation with elastic bands to ensure a correct elevation of the lesion. Before proceeding with the resection, a distribution study was conducted by means of an abdominopelvic CT scan, a test for urinary 5-hydroxyindoleacetic acid, or an octreotide scintigraphy.
Patients considered suitable for endoscopic resection were those with tumors measuring less than 1 cm in diameter in whom an endoscopic ultrasonography had shown that the fourth layer (the muscularis propria) had not been affected by the tumor, and those in whom extension studies ruled out distant spread. Some patients who exhibited excessive surgical risk, who had larger tumors even with still localized disease, were also subjected to endoscopic resection. Finally, after resection, follow-up was scheduled as consisting of eschar biopsies at one and twelve months after tumor removal.
Variables studied
In addition to demographic data, the study included all the variables related to the endoscopic and echoendoscopic characteristics of the lesions (size, morphology, edge appearance, echogenicity, presence of cystic areas or hyperechogenic foci, presence of adenopathies, ulcerations, and layer in which the tumors were found). Similarly, we made a detailed study of the location of lesions, clinical manifestations derived from them, reason why resection was indicated, and resection technique used. Logically, the primary endpoint of the study was to evaluate the effectiveness of endoscopic resection for submucosal tumors (completeness of resection) and the safety of this technique (presence and nature of complications). For the statistical analysis, we used the SPSS 11.5 statistical software package for Windows.
RESULTS
During the study period, 24 carcinoid tumors were treated endoscopically in 21 patients (19 patients with single tumors, two with multicentric tumors). Patient mean age was 51.76 years (range, 31 to 73), and the majority of patients were males (71.5%).
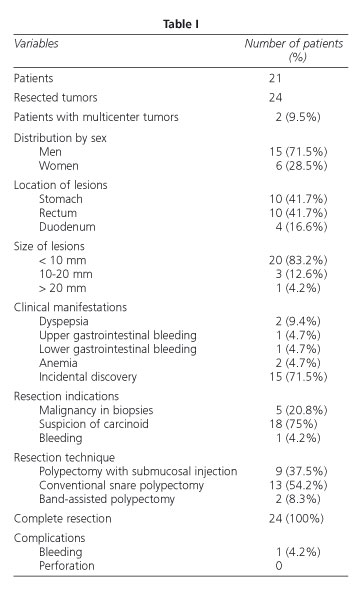
The majority of tumors were gastric lesions (41.7%) or rectal lesions (41.7%). The remaining lesions were located in the duodenum (16.6%). In most cases (71.5%), tumors were incidental findings arising during examinations indicated for other reasons (Table I).
The average size of the lesions measured by conventional endoscopy was 8 mm, significantly smaller (p = 0.02) than the size of the resection piece (9 mm). However, the average size measured by EUS was 8.95 mm, with no significant differences with respect to the size measured by a pathologist (p = 0.25). In all, 83.2% of lesions (20 tumors) measured less than 10 mm, three lesions measured between 10 and 20 mm, and only one was greater than 20 mm. In all cases, localized disease was confirmed; in patients with lesions larger than 10 mm, surgical risk from associated diseases was too high.
In all of the cases, lesions depended from the muscularis mucosae, had regular edges, were hypoechogenic, and had homogeneous echographic pattern (Fig. 1). In only one case, the tumor was ulcerated and was the cause of digestive bleeding. A resection of submucosal lesions was indicated in 18 cases (75%) following suspicion of a carcinoid tumor as arisen by ultrasonography, in five cases (20.8%) by biopsies suggesting malignancy, and in another case by tumor-related bleeding (Table I).
In 13 cases (54.2%), the resection was performed using the conventional snare polypectomy technique; in 9 cases (37.5%), the resection was preceded by injection of physiological saline and/or adrenaline to achieve better elevation of the lesion; and finally, in 2 cases (8.3%) the resection was done after ligating the lesion with elastic bands (Fig. 2). Latter cases involved very small lesions measuring 5 and 6 mm that could not be properly elevated by submucosal injection.
In all cases, the resection was complete; this result was documented by examining the resection piece, which showed tumor-free edges; furthermore, biopsies performed 30 days and 12 months after resection ruled out local recurrence in all cases. Only one important complication arose: a significant hemorrhage that was controlled endoscopically. The patient was hospitalized for observation, and did not require blood transfusion.
DISCUSSION
Endoscopic resection of carcinoid tumors is not a new technique; it has been used since the 1990s. In 1997, Higaki et al. (39) published a series of 22 rectal carcinoids, 18 of which were endoscopically resected. The authors showed that when a tumor was less than 10 mm in diameter and did not invade tissue beyond the submucosa (muscularis propria spared), the risk of distant spread was minimal, which indicated that, in such cases, local endoscopic resection was a valid alternative. We have followed these criteria to indicate an endoscopic resection in lesions of this nature: lesions having a diameter less than 10 mm, with evidence of local disease, and a tumor-free muscular layer. Only four larger lesions were endoscopically resected; in all cases, the extension study was negative, and the high surgical risk made local resection the most suitable procedure. Also in 1997, Imada-Shirakata et al. (40), and more recently Oshitani et al. (41), reached the same conclusions, albeit with a series of only 8 and 7 patients, respectively. Both the size of the tumor and the wall layer in which it is located can be established with high accuracy using EUS; this is also true for the rest of submucosal tumors (26,27,32). In our experience, EUS enabled us to successfully establish the origin layer in all cases, and to evaluate very precisely the size of the tumor. On the other hand, conventional endoscopy underestimates lesion size as it can only evaluate intraluminal growth.
From an ultrasonographic standpoint, the morphology of carcinoid tumors is quite similar to that of leiomyomas: well-delimited, hypoechoic lesions with homogeneous echogenicity, regular edges, and located in the first three layers of the intestinal wall (11). Thus, in many cases, resection is the only way to reach a diagnosis. However, the endoscopic resection of submucosal lesions has a well-documented complication rate, being the bleeding the most common of them. Its frequency varies from 4.8 to 16% (20-23), and it was endoscopically solved in all reported cases, with no need for surgery. Only one major complication occurred in our series (1 case of bleeding controlled endoscopically); it must be borne in mind that nearly all of the lesions were located in the gastric cavity and in the rectum, where the frequency of complications is significantly less than in the right colon or even the duodenum. EUS is the most suitable technique for evaluating size of tumors and their exact location in the inner wall of the gastrointestinal tract, and even to venture an opinion on the lesion's nature. Additionally, some authors have also used ultrasonography to show the correct elevation of lesions after submucosal injection of various substances (41). In contrast, Lachter et al. (43) stated that endoscopic ultrasonography may have a limited value because, in their experience, a prior echoendoscopic study does not exclude subsequent follow-up and resection margins were infiltrated by the tumor in all their cases. However, their experience is limited to only three tumors, and their article mentions some other important aspects of EUS, such as its ability to exclude the presence of vascular structures near the lesion that otherwise would markedly increase the risk of hemorrhage. Consequently, even if the first of their statements is accepted, the second would concede an enormous value to EUS-assisted endoscopic resection of submucosal tumors.
Different techniques have been described for carcinoid tumor resection. Resection may be performed by using the conventional snare polypectomy technique, but some authors prefer a previous elevation of the lesion by means of submucosal injection of various substances, such as physiological saline, diluted adrenaline, etc. (42,44). Other authors have described a technique involving aspiration of the lesion into a cap, as in elastic band ligation, which would facilitate the grasping of the lesion with a polypectomy snare (40,41). Some physicians have proposed elevation of the lesions by ligating them with elastic bands to ease their removal using a polypectomy snare (45-48). Kajiyama et al. (47) reported that the use of the aspiration method, either with elastic band ligation or a cap, is clearly more effective than the strip biopsy method; they indicated that aspiration ensures diagnosis in 95% of cases, compared to 77% with strip biopsy, and that a complete resection of the lesion is achieved in 87% of cases involving aspiration, compared to 74% for strip biopsy. Recently, Ono et al. (48) published a study in which they compared two resection techniques. They described a series of 14 rectal carcinoid tumors resected using an elastic band-assisted resection technique, achieving complete resection in 100% of cases. These results were compared with those of a retrospective series of 14 cases resected using a conventional snare polypectomy technique or a mucosectomy technique, in which resection margins were affected in 42% of cases. Differences exist even within this group, since surgical margins were affected less frequently in patients undergoing mucosectomy. These findings differ markedly from the results in our series. In 13 cases, we employed a snare polypectomy technique, in nine a resection assisted by submucosal injection of physiological saline and/or diluted adrenaline, and in two cases a resection assisted by elastic bands. The resection was complete in 100% of cases, and in no case did we observe local or distant recurrences (all extension studies had also been negative). Therefore, we cannot agree with these findings of these authors, since our results are radically different and few conclusions can be drawn from a single, uncontrolled study.
In summary, endoscopic resection of carcinoid tumors in the digestive tract is a valid alternative in the treatment of these lesions. Moreover, this technique must be supported by EUS, since this is the most precise diagnostic technique for evaluating tumor size, and the best suited method for showing the tumor-free state of the muscularis propria. These two factors seem to condition the possibility of distant metastasis. Obviously, it is vital that distant tumor spread be ruled out before proceeding with the resection procedure.
REFERENCES
1. Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001; 92 (8): 2204-10. [ Links ]
2. Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997; 79 (4): 813-29. [ Links ]
3. Ballantyne GH, Savoca PE, Flannery JT, Ahlman MH, Modlin IM. Incidence and mortality of carcinoids of the colon. Data from the Connecticut Tumor Registry. Cancer 1992; 69 (10): 2400-5. [ Links ]
4. Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy 1997; 29 (3): 171-5. [ Links ]
5. Koura AN, Giacco GG, Curley SA, Skibber JM, Feig BW, Ellis LM. Carcinoid tumors of the rectum: effect of size, histopathology, and surgical treatment on metastasis free survival. Cancer 1997; 79 (7): 1294-8. [ Links ]
6. Yoshida M, Tsukamoto Y, Niwa Y, Goto H, Hase S, Hayakawa T, et al. Endoscopic assessment of invasion of colorectal tumors with a new high-frequency ultrasound probe. Gastrointest Endosc 1995; 41 (6): 587-92. [ Links ]
7. Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg 1994; 179 (2): 231-48. [ Links ]
8. Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999; 340 (11): 858-68. [ Links ]
9. Arguello L, Pellise M, Miquel R. Utility of echoendoscopy in the evaluation of submucosal tumors and extrinsic compressions of the digestive tract. Gastroenterol Hepatol 2002; 25 (1): 13-8. [ Links ]
10. Miettinen M, Lasota J. Gastrointestinal stromal tumors-definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001; 438 (1): 1-12. [ Links ]
11. Chak A. EUS in submucosal tumors. Gastrointest Endosc 2002; 56 (Supl. 4): S43-8. [ Links ]
12. Catalano MF. Endoscopic ultrasonography in the diagnosis of submucosal tumors: need for biopsy. Endoscopy 1994; 26 (9): 788-91. [ Links ]
13. Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 1995; 27 (2): 171-7. [ Links ]
14. Gu M, Ghafari S, Nguyen PT, Lin F. Cytologic diagnosis of gastrointestinal stromal tumors of the stomach by endoscopic ultrasound-guided fine-needle aspiration biopsy: cytomorphologic and immunohistochemical study of 12 cases. Diagn Cytopathol 2001; 25 (6): 343-50. [ Links ]
15. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997; 112 (4): 1087-95. [ Links ]
16. Dodd LG, Nelson RC, Mooney EE, Gottfried M. Fine-needle aspiration of gastrointestinal stromal tumors. Am J Clin Pathol 1998; 109 (4): 439-43. [ Links ]
17. Brainard JA, Goldblum JR. Stromal tumors of the jejunum and ileum: a clinicopathologic study of 39 cases. Am J Surg Pathol 1997; 21 (4): 407-16. [ Links ]
18. Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, et al. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc 2002; 55 (1): 44-9. [ Links ]
19. Kawamoto K, Yamada Y, Furukawa N, Utsunomiya T, Haraguchi Y, Mizuguchi M, et al. Endoscopic submucosal tumorectomy for gastrointestinal submucosal tumors restricted to the submucosa: a new form of endoscopic minimal surgery. Gastrointest Endosc 1997; 46 (4): 311-7. [ Links ]
20. Kojima T, Takahashi H, Parra-Blanco A, Kohsen K, Fujita R. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc 1999; 50 (4): 516-22. [ Links ]
21. Hyun JH, Jeen YT, Chun HJ, Lee HS, Lee SW, Song CW, et al. Endoscopic resection of submucosal tumor of the esophagus: results in 62 patients. Endoscopy 1997; 29 (3): 165-70. [ Links ]
22. Wei SC, Wong JM, Shieh MJ, Sun CT, Wang CY, Wang TH. Endoscopic resection of gastrointestinal submucosal tumors. Hepatogastroenterology 1998; 45 (19): 114-8. [ Links ]
23. Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 2003; 57 (1): 68-72. [ Links ]
24. Palazzo M, Rouseau G. Écho-endoscopie digestive. Paris: Masson; 1998: 21-7. [ Links ]
25. Shen EF, Arnott ID, Plevris J, Penman ID. Endoscopic ultrasonography in the diagnosis and management of suspected upper gastrointestinal submucosal tumours. Br J Surg 2002; 89 (2): 231-5. [ Links ]
26. Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut 2000; 46 (1): 88-92. [ Links ]
27. Rosch T. Endoscopic ultrasonography in upper gastrointestinal submucosal tumors: a literature review. Gastrointest Endosc Clin N Am 1995; 5 (3): 609-14. [ Links ]
28. Rosch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, et al. German EUS Club. Endoscopic ultrasonography. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol 2002; 37 (7): 856-62. [ Links ]
29. Brand B, Oesterhelweg L, Binmoeller KF, Sriram PV, Bohnacker S, Seewald S, et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis 2002; 34 (4): 290-7. [ Links ]
30. Souquet JC, Bobichon R. Role of endoscopic ultrasound in the management of the submucosal tumors in the esophagus and the stomach. Acta Endoscop 1996; 26: 307-12. [ Links ]
31. Gress F, Schmitt C, Savides T, Faigel DO, Catalano M, Wassef W, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc 2001; 53 (1): 71-6. [ Links ]
32. Takada N, Higashino M, Osugi H, Tokuhara T, Kinoshita H. Utility of endoscopic ultrasonography in assessing the indications for endoscopic surgery of submucosal esophageal tumors. Surg Endosc 1999; 13 (3): 228-30. [ Links ]
33. Kameyama H, Niwa Y, Arisawa T, Goto H, Hayakawa T. Endoscopic ultrasonography in the diagnosis of submucosal lesions of the large intestine. Gastrointest Endosc 1997; 46 (5): 406-11. [ Links ]
34. Geller A, Wang KK, DiMagno EP. Diagnosis of foregut duplication cysts by endoscopic ultrasono-graphy. Gastroenterology 1995; 109 (3): 838-42. [ Links ]
35. Bhutani MS, Hoffman BJ, Reed C. Endosonographic diagnosis of an esophageal duplication cyst. Endoscopy 1996; 28 (4): 396-7. [ Links ]
36. Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, et al. Endosonographic features of esophageal granular cell tumors. Endoscopy 1997; 29 (9): 850-3. [ Links ]
37. Hizawa K, Iwai K, Esaki M, Suekane H, Inuzuka S, Matsumoto T, et al. Endosonographic features of Brunner's gland hamartomas which were subsequently resected endoscopically. Endoscopy 2002; 34 (12): 956-8. [ Links ]
38. Chak A, Canto MI, Rosch T, Dittler HJ, Hawes RH, Tio TL, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc 1997; 45 (6): 468-73. [ Links ]
39. Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy 1997; 29 (3): 171-5. [ Links ]
40. Imada-Shirakata Y, Sakai M, Kajiyama T, Kin G, Inoue K, Torii A, et al. Endoscopic resection of rectal carcinoid tumors using aspiration lumpectomy. Endoscopy 1997; 29 (1): 34-8. [ Links ]
41. Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, Matsumoto T, et al. Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res 2000; 28 (5): 241-6. [ Links ]
42. Nishimori I, Morita M, Sano S, Kino-Ohsaki J, Kohsaki T, Suenaga K, et al. Endosonography-guided endoscopic resection of duodenal carcinoid tumor. Endoscopy 1997; 29 (3): 214-7. [ Links ]
43. Lachter J, Chemtob J. EUS may have limited impact on the endoscopic management of gastric carcinoids. Int J Gastrointest Cancer 2002; 31 (1-3): 181-3. [ Links ]
44. Yoshikane H, Suzuki T, Yoshioka N, Ogawa Y, Hamajima E, Hasegawa N, et al. Duodenal carcinoid tumor: endosonographic imaging and endoscopic resection. Am J Gastroenterol 1995; 90 (4): 642-4. [ Links ]
45. Akahoshi K, Fujimaru T, Nakanishi K, Harada N, Nawata H. Endosonography probe-guided endoscopic resection of small flat rectal carcinoid tumor using band ligation technique. Endoscopy 2001; 33 (5): 471. [ Links ]
46. Vázquez-Iglesias JL, Souto Ruzo J. A new technique for endoscopic resection of rectal carcinoid tumors. Digestion 1998; 59 (Supl. 3): 542. [ Links ]
47. Kajiyama T, Hajiro K, Sakai M, Inoue K, Konishi Y, Takakuwa H, et al. Endoscopic resection of gastrointestinal submucosal lesions: a comparison between strip biopsy and aspiration lumpectomy. Gastrointest Endosc 1996; 44 (4): 404-10. [ Links ]
48. Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, Saito D. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc 2003; 57 (4): 583-7. [ Links ]











 texto en
texto en