Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.2 Madrid feb. 2005
| ORIGINAL PAPERS |
Effect of ethanol consumption on colon cancer in an experimental model
S. Pérez-Holanda, L. Rodrigo1, J. Viñas-Salas2 and C. Piñol-Felis2
Department of Surgery. Hospital Grande Covián. Arriondas. Asturias. 1Department of Medicine. Faculty of Medicine.
University of Oviedo. Asturias. 2Department of Medicine and Department of Surgery. Faculty of Medicine.
University Hospital Arnau de Vilanova. University of Lleida. Spain
ABSTRACT
Aims: the present study was designed to examine the effect of an ethanol supplement on experimental colon carcinogenesis.
Material and methods: one hundred and ten 10-week-old Sprague-Dawley rats were divided into five groups: group A (20 rats) received no treatment. Group B (20 rats) received a supplement of ethanol at 1.23 g/kg of body weight per day added to their drinking water for 24 weeks. Group C (30 rats) received 18 weekly doses of dimethylhydrazine (DMH) at 21 mg/kg of body weight from the beginning of the study. Group D (20 rats) received ethylen-diamin-tetracetic acid (EDTA) solution only for 18 weeks. Group E (20 rats) received ethanol at the same dose as group B plus DMH injections at the same dose as the rats in group C from the beginning of the study. All experimental animals were sacrified after 25-27 weeks.
Results: no significant differences in the number of rats that developed tumors, number of tumor-free animals, and number of tumors per rat, as well as in macro-microscopic tumoral findings were observed for animals in group C compared to animals in group E.
Conclusions: we concluded that the addition of an ethanol supplement does not modify colorectal carcinogenesis using a dynamic model of tumor induction with DMH.
Key words: Alcohol. Cancer. Carcinogenesis. Colon. Dimethylhydrazine. Ethanol. Neoplasm. Tumors.
Pérez-Holanda S, Rodrigo L, Viñas-Salas J, Piñol-Felis C. Effect of ethanol consumption on colon cancer in an experimental model. Rev Esp Enferm Dig 2003; 95: 87-96.
Recibido: 09-01-04.
Aceptado: 14-07-04.
Correspondencia: S. Pérez-Holanda. Castiello, 22, 33394 Gijón. Asturias. Tel.: 985 132 948. Fax: 985 134 108. e-mail: direccionmedica.gae6@sespa.princast.es
INTRODUCTION
Colorectal cancer (CRC) is a frequent tumor with a high mortality in western countries (1), where alcohol intake is a common habit. In meta-analyses, environmental factors play a role in the promotion of this tumor (2). In fact, its incidence rate could be reduced approximately by 30-35% by modifying dietary habits (3).
In an important paper related to epidemiologic evidence published in 1992 (4), an association between alcohol consumption and CRC was reported. More recently, while some clinical studies support that ethanol intake increases the risk of CRC (5-8), other report no relationship (9,10), while others suggest adenoma-promoting effects only (11). It is difficult to perform a long-term prospective study on the influence of different environmental factors in human colon cancer (12). Nevertheless, they report some contradictory results, supporting cancer promotion (13) or adenoma promotion only (14). On other hand, experimental studies have shown an increased risk of CRC due to the effect of alcohol both in vitro (15) and in rodent models (16,17).
Colonic carcinogenesis induced with 1,2 dimethylhydrazine (DMH) in rats has proven to be a close and valuable experimental model of human disease because DMH-induced tumors resemble human colorectal cancer both macro-microscopically and in their clinical behavior (18-20).
The aim of the present study is to investigate the effect of ethanol consumption on experimental colon carcinogenesis using a dynamic model with concomitant administration of alcohol and DMH.
MATERIAL AND METHODS
Previous studies (21,22) have shown that the number of tumor-free animals was 10-24%, and the number of tumors per rat was 1.87. The mortality rate was high in this experimental model (23,24). Other studies report the same allocation in the number of animals in each group (25).
One hundred and ten 10-week-old Sprague-Dawley rats (Lab. Letica®, Barcelona, Spain), 55 males and 55 females of identical strain, were allocated to one of five groups with equal gender distribution. The 20 rats in group A (control group) received no treatment. The 20 rats in group B (ethanol control group) received ethanol at 1.23 g/kg of body weight (wt) per day added to the drinking water from the beginning until the conclusion of the experiment. Group C was composed of 30 rats due to the high mortality rate obtained in previous studies (22,24). These rats in group C (ethanol control group) were injected subcutaneously (s.c.) with 18 weekly doses of 21 mg/kg of body wt of 1,2 dimethylhydrazine (DMH; Fluka Chemica A.G., Sigma Co.®, St. Louis, Missouri, U.S.A.) from the beginning of the study to week 27. DMH was prepared, as in previous studies, using a dilution of 400 mg of DMH in distilled water, containing 37 mg of ethylenediaminetetracetic acid (EDTA; Farmitalia Carlo Erba S.P.A.®, Milano, Italy) solution as a stabilizing agent, and using 0.1 M sodium hidroxyde buffer for pH 6.5 (22). The solution was prepared weekly prior to injection into the lumbar region of the rat. The 20 rats in group D (EDTA control group) received the same volume of EDTA solution only. The 20 rats in group E (DMH + ethanol group) were treated with 18 weekly s.c. injections of 21 mg of DMH/kg of body wt, and 1.23 g/kg per day of ethanol added to the drinking water, beginning at the same time as the first weekly injection of DMH and up to the end of the study. Daily ethanol intake was quantified by controlling daily drinking water consumption.
All rats were fed on standard rodent diet (ITM-R20, Lab. Letica®, Barcelona, Spain) with 3% fat and 5% starch. Daily food consumption by treated groups was controlled throughtout the study. Fifty percent of animals were weighed weekly until sacrificed. The dose of ethanol given to rats was strictly controlled in order to prevent dehydration when adding alochol to drinking water as observed by others authors (26). Temperature and humidity were controlled throughout the study period in the room where animals were kept, with a 12-hour light schedule (27,28). Animals were lodged in cages with a maximum number of three per cage in order to avoid cannibalism, and to control oral consumption of ethanol as in previous studies (22-24).
European Ethical Committee's recommendations (E. E. C. Directives 1986/609) were followed throughout the study.
Surviving rats were sacrificed at weeks 25 to 27 with a lethal intraperitoneal innoculation of 4.5% chloral hydrate. In order to avoid the time effect variability, a fixed number of rats from each group were sacrificed during the same week (22).
At autopsy, thoracic and abdominal cavities were examined. The colon and rectum were removed, opened along the antimesenteric border, and gently cleaned of residue with water. The entire gastrointestinal tract was palpated for tumors, adhesions or other abnormalities. The number of tumors, and their location and size were all recorded. Tumors and normal colonic mucosa specimens were taken from the cecum and ascending (right) colon, and specimens from the transverse and descending colon and rectum (left colon) were removed and fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological observation. Mucosal lesions were classified following criteria by Grau de Castro-Piqué Badía (29) and Lev (30). Tumors obtained were classified according to extent of invasion and differentiation, morphology (considering a mucinous carcinoma when the mucinous component was > 50%), tumor size and macroscopic appearance, and the presence of associated lymphoid tissue. Small-bowel tumors and other extraintestinal findings were also noted.
A Chi square test was performed on the DMH and DMH + ethanol groups in order to compare tumor incidence, anatomopathological findings, total colorectal tumors, left versus right colon tumor totals, average tumor size, and tumor association with lymphoid tissue. In the event that conditions for the application of the Chi square test were not fulfilled, Fisher's exact test was used. The differences between both groups were significant when the p value was less than or equal to 0.05. Results are presented as mean (average) ± standard deviation (SD).
RESULTS
Six rats (5.5%) died before completion of the study, two in the DMH group (C; 6.7%), and four in the DMH + ethanol group (E; 20.0%). In group C one male rat died without the possibility of an early autopsy, and one female rat showed no tumors when the autopsy was performed. In group E, two male rats died due to an intestinal obstruction by a colon tumor in both cases; one male rat developed a colon tumor with metastases and digestive hemorrhage; and one female rat showed no tumors. These animals were therefore excluded from the analysis. The rats that completed the study showed no evidence of dehydration.
Alcohol consumption was similar in both ethanol-fed groups (1.23 ± 0.0074 g/kg of body wt per day in group B compared to 1.23 ± 0.0094 in group E; p = 0.82), ethanol consumption was measured daily throughout the study.
No tumors were seen in the animals of DMH-free groups (A, B, and D). Tumors developed only in DMH-treated groups: twenty-five rats in the DMH group (28 rats) and sixteen rats in the DMH + ethanol group (16 rats). With respect to gender, there were fewer tumor-free animals amongst males (14.3% in both groups) versus females (78.6% in the DMH group, p = 0.0006; 55.6% in the DMH + ethanol group, p = 0.14, Fisher's exact test) (Table I). When excluding tumor-free animals, no differences were observed in the mean number of tumors per rat (1.67 in the DMH group compared to 1.60 in the DMH + ethanol group). No hepatic metastases were recorded in either group.
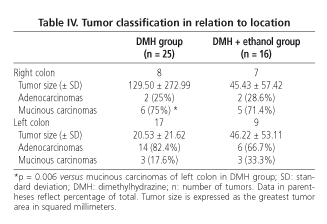
No significant differences were found in tumor size (55.40 mm2 in the DMH group compared toh 45.87 mm2 in the DMH + ethanol group). In terms of macroscopic morphology, 80% of tumors had a polypoid aspect in the DMH group, versus 81.3% in the DMH + ethanol group. In relation to tumor location, 68% of tumors were located in the left colon in the DMH group, compared to 56.2% in the DMH + ethanol group (Table II).
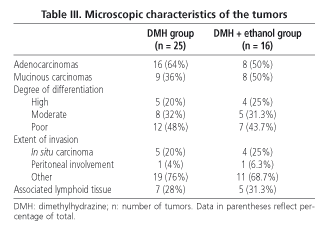
Microscopic findings (Table III) revealed no differences for animals in the DMH group compared to those in the DMH + ethanol group in terms of number of adenocarcinomas and mucinous carcinomas (considering a mucinous component > 50% of tumor), or regarding extent of invasion or differentiation, or in tumor association with lymphoid tissue. However, DMH-treated rats in group C (DMH control group) had a significantly higher number of mucinous carcinomas in the right colon (75%) compared to those found in the left colon (17.6%; p = 0.006); in the DMH + ethanol group this trend was also observed (71.4% in the right colon versus 33.3% in the left colon, p = 0.31 using Fisher's exact test) (Table IV).
Two small-bowel tumors were also found: one in the DMH group and one in the DMH + ethanol group. A tumor of the ear was found in the DMH group.
DISCUSSION
Ethanol consumption is described as a risk factor for colorectal cancer, a hypothesis supported by both clinical (5-8) and experimental trials in rats (16,17).
To facilitate colon cancer studies with controlled variables, chemically induced carcinogenesis with 1,2 dimethylhydrazine (DMH) in the rat has proven to be a valuable experimental model for more than three decades (18-20).
In retrospective studies, an increased risk for colon cancer has been described for women at a dose of 63 g per day (5). Other authors (9) found no relationship at 30 g per day. Following these clinical findings, our rats received 1.23 g/kg of ethanol daily for 24 weeks, which is a common but risky consumption in humans. We present an original work where a common alcohol consumption, at a fixed dose and duration, is used to prove the absence of influence in colorectal carcinogenesis.
Under these conditions and with the amount of ethanol described, rats showed no natural aversion to alcohol. Thus, we did not expect to find significant differences in ethanol consumption between the DMH + ethanol group and the non-ethanol control group.
Depending on experimental conditions, chemically induced colorectal carcinogenesis is controversial: when DMH is given in the presence of alcohol carcinogenesis is inhibited, whereas in the absence of alcohol carcinogenesis is stimulated due to an ethanol-associated microsomal cytochrome P450 induction (31). Moreover, when using a primary carcinogen instead of a procarcinogen, alcohol stimulates carcinogenesis (32). Based on the results of these studies the administration of alcohol could have decreased DMH-induced carcinogenesis; however, in our study no significant differences were found in colorectal carcinogenesis when comparing the DMH group to the DMH + ethanol group.
The dose of ethanol administered did not significantly modify the number of DMH-induced tumor-free rats, nor did it modify the number of tumors per rat either. Some authors (32) have described no significant differences in tumor size or in tumor histology due to an alcohol effect, as found in our study. Nevertheless, these authors have shown an increased number of tumors in the rectum, but this was not seen in our rats. We found that ethanol consumption did not alter the number or size of DMH-induced tumors, or their distribution throughout the colorectal tract, this being similar to findings by other authors in DMH models (33).
In human studies, patients with colorectal carcinoma who use alcohol seem to have higher cancer-related mortality rates due to a greater development of liver metastases (34). No support for these findings was found in our study.
In vitro studies (15) have described that ethanol consumption may enhance the aggressiveness of colorectal tumors. These authors defined it as increased tumoral cell proliferation rate, decreased tumoral differentiation, and diminished cellular adhesion. Although some studies in mice (35) report that ethanol intake promotes changes in the mucosal lymphoid tissue, and that this may explain the cocarcinogenesis mechanism (28), this was not observed in our rats. Our results show no significant differences in the anatomopathologic characteristics of tumors in DMH-induced rats as a result of the effect of alcohol. Thus, this aggressive behavior was not detected in tumors found in our animals.
In epidemiologic studies, the mechanism by which alcohol intake may modify carcinogenesis seems to be time- and dose-response related (4,5,36), although some studies are far from unanimous on this issue (37). Taking these studies together, data suggest that the longer the ethanol intake, the lower the dose per day needed. Following this hypothesis, the reason for our findings -which suggest that ethanol would not act in the pathogenic mechanism of colon cancer- may lie in a timing effect variability; it seems that this relatively low dose is not dangerous enough to modify colorectal carcinogenesis, but a dynamic model of concomitant administration of alcohol and procarcinogen, as used by us, is a controversial design for studying colorectal carcinogenesis as induced by ethanol.
Certain authors have shown a spontaneous colonic carcinogenesis rate of less than 2-3 rats per 100.000 animals (38). In our DMH-free rats, and as expected, no tumors developed.
Tumors in the proximal and distal colon and rectum must be considered to have some differences regarding risk factors, etiology, histological appearance, and growth characteristics of tumors; this is based on epidemiologic studies (9) and experimental studies in rats (39); thus responses of the epithelium may differ at different locations in DMH-induced rats (16).
It is important to note that tumors in our rats were classified according to the criteria described above. According to these, a mucinous adenocarcinoma is considered a poorly differentiated tumor (29). A correlation was found between tumors located in the right colon and the poor grade of differentiation, both in experimental studies (40) and literature reviews (41). In our study, we have been able to demostrate the existence of an increased number of mucinous carcinomas in the right colon compared to the left colon in rats included in the DMH group, although a trend towards this behaviour has been seemingly shown in rats in the DMH + ethanol group (probably because the number of tumors was not significant enough). Our data are in conflict with results from other studies (31,42), which have reported that the number of right colonic tumors was inversely correlated with alcohol consumption in a dynamic model of carcinogenesis. We therefore think that the dose of alcohol in our study was relatively low to show any inhibitory effect on right colonic tumors; right tumors therefore showed their own and worse behavior when compared to left tumors, as suggested by some authors (40,41). Our results are not comparable to those by authors (25,43) due to the different histologic criteria used (29,44).
Firstly we conclude that an oral ethanol supplement at a dose of 1.23 g/kg of body wt per day for 24 weeks does not modify colon cancer in a dynamic model of carcinogenesis induced by DMH in Sprague-Dawley rats; and secondly that a different histologic behavior exists in tumors located in the right colon as compared to tumors located in the left colon due to the effect of ethanol.
ACKNOWLEDGEMENTS
The authors wish to thank Mr. Manuel Santiago for his technical support and animal care. This research work has been supported by University of Lleida, and a grant "Research Help FIS number 89/0612".
REFERENCES
1. Greenwald P. Colon cancer overview. Cancer 1992; 70: 1206-15. [ Links ]
2. Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables and colon cancer: Critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst 1990; 82: 650-61. [ Links ]
3. Vargas PA, Alberts DS. Primary prevention of colorectal cancer through dietary modifications. Cancer 1992; 70: 1229-35. [ Links ]
4. Kuhne GA, Vitetta, L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer 1992; 18: 97-111. [ Links ]
5. Barra S, Negri E, Franceschi S, Guarneri S, La Vecchia C. Alcohol and colorectal cancer: A case-control study from northern Italy. Cancer Causes Control 1992; 3: 153-9. [ Links ]
6. Glynn SA, Albanes D, Pietinen P, Brown CC. Rautalahti M, Tangrea JA, et al. Alcohol consumption and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1996; 7: 214-23. [ Links ]
7. Murata M, Takayama K, Choi BC, Pak AW. A nested case-control study on alcohol drinking, tobacco smoking, and cancer. Cancer Detect Prev 1996; 20: 557-65. [ Links ]
8. Yamada K, Araki S, Tamura M, Sakai I, Takahashi Y, Kashihara, et al. Case-control study of colorectal carcinoma in situ and cancer in relation to cigarette smoking and alcohol use (Japan). Cancer Causes Control 1997; l 8: 780-5. [ Links ]
9. Gerhardsson de Verdier M, Romelsjo A, Lundberg M. Alcohol and cancer of the colon and rectum. Eur J Cancer Prev 1993; 2: 401-8. [ Links ]
10. Tavani A, Ferraroni M, Mezzetti M, Franceschi S, Lo Re A, La Vecchia C. Alcohol intake and risk of cancers of the colon and rectum. Nutr Cancer 1998; 30: 213-9. [ Links ]
11. Kono S, Ikeda N, Yanai F, Shinchi K, Imanishi K. Alcoholic beverages and adenomatous polyps of the sigmoid colon: A study of male self-defence officials in Japan. Int J Epidemiol 1990; 19: 848-52. [ Links ]
12. Weisburger JH. Causes, relevant mechanisms and prevention of large bowel cancer. Semin Oncol 1991; 18: 316-36. [ Links ]
13. Goldbohm RA, Van den Brandt PA, Van´t Veer P, Dorant E, Stumans F, Hermus RJ. Prospective study on alcohol consumption and the risk of cancer of the colon and rectum in the Netherlands. Cancer Causes Control 1994; 5: 95-104. [ Links ]
14. Nagata C, Shimizu H, Kametani M, Takayama N, Ohnuma T, Matsushita S. Cigarette's smoking, alcohol use, and colorectal adenoma in Japanese men and women. Dis Colon Rectum 1999; 42: 337-42. [ Links ]
15. KoivistoT, Salaspuro M. Acetaldehyde alters proliferation, differentiation, and adhesion properties of human colon adenocarcinoma cell line Caco-2. Carcinogenesis 1998; 19: 2031-6. [ Links ]
16. Seitz HK, Czygan P, Simanowski UA, Waldherr R, Veith S, Raedsch R, et al. Stimulation of chemically induced rectal carcinogenesis by chronic ethanol ingestion. Alcohol 1985; 20: 427-33. [ Links ]
17. Simanowski UA, Stickel F, Maier H, Gartner U, Seitz HK. Effects of alcohol on gastrointestinal cell regeneration as a possible mechanism in alcohol-associated carcinogenesis. Alcohol 1995; 12: 111-5. [ Links ]
18. Banerjee A, Quirke P. Experimental models of colorectal cancer. Dis Colon Rectum 1998; 41: 490-505. [ Links ]
19. Barkla DH, Tutton PJ. Ultrastructure of 1,2-dimethylhydrazine-induced adenocarcinomas in rat colon. J Natl Cancer Inst 1997; 61: 1291-9. [ Links ]
20. Druckrey H, Preussman R, Matzkies F. Selektive Erzeugung von Darmkaebs bei Ratten durch 1,2-Dimethylhydrazin. Naturwissenschaften 58:285-286; 1967. In: Barkla DH, Tutton PJ. Ultrastructure of 1,2-dimethylhydrazine-induced adenocarcinomas in rat colon. J Natl Cancer Inst 1988; 61: 1291-9. [ Links ]
21. Hupp T, Buhr HJ, Ivancovic S, Beck N. Tierexperimentelle Untersuchungen zur karzinogeninduzierten Tumorbildung an Kolotomien in Abhängigkeit von Nahtmaterialien (Influence of suture material on experimental colonic carcinogenesis in rats). Langenbecks Arch Chir 1992; 377: 9-13. [ Links ]
22. Viñas Salas J, Biendicho Palau P, Piñol Felis C, Miguelsanz García S, Pérez Holanda S. Calcium inhibits colon carcinogenesis in an experimental model in the rat. Eur J Cancer 1998; 34: 1941-5. [ Links ]
23. Buenestado García J, Reñé Espinet JM, Piñol Felis C, Viñas Salas J. Modelo experimental de cáncer colorrectal. Rev Esp Enferm Dig 2002; 94: 367. [ Links ]
24. Buenestado García J, Piñol Felis C, Reñé Espinet JM, Pérez-Holanda S, Viñas Salas J. Silk suture promotes colon cancer in an experimental carcinogenic model. Dig Liver Dis 2002; 34: 609-10. [ Links ]
25. Noguera Aguilar JF, Tortajada Collado C, Moron Canis JM, Plaza Martínez A, Amengual Antich I, Pujol Tugores JJ. Modelo experimental para el estudio de la recidiva locorregional del cáncer colorrectal. Rev Esp Enferm Dig 2002; 94: 131-8. [ Links ]
26. Seitz HK, Simanowski UA. Alcohol and carcinogenesis. Am Rev Nutr 1988; 8: 99-119. [ Links ]
27. Glauert HP, Bennink MR. Influence of diet or intrarectal bile acid injections on colon epithelial cell proliferation in rats previously injected with 1,2-dimethylhydrazine. J Nutr 1983; 113: 475-82. [ Links ]
28. Hardman WE, Cameron IL. Colonic crypts located over lymphoid nodules of 1,2-dimethylhydrazine-treated rats are hyperplastic and at high risk of forming adenocarcinomas. Carcinogenesis 1994; 15: 2353-61. [ Links ]
29. Grau de Castro JJ, Piqué Badía JM. Cáncer colorrectal. En: Monografías clínicas en oncología. Barcelona: Ed. Doyma SA, 1990; 8: 63-75. [ Links ]
30. Lev R. Adenomatous polyps of the colon: Pathological and clinical features. New York: Springer-Verlach Inc., 1990. p. 1-2, 1-18. [ Links ]
31. Seitz HK, Czygan P, Waldherr R, Veith S, Raedsch R, Kässmodel H, et al. Enhacement of 1,2-Dimethylhydrazine-induced rectal carcinogenesis following chronic ethanol consumption in the rat. Gastroenterology 1984; 86: 886-91. [ Links ]
32. Seitz HK, Simanowski UA, Garzon FT, Rideout JM, Peters TJ, Koch A, et al. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology 1990; 98: 406-13. [ Links ]
33. McGarrity TJ, Peiffer LP, Colony PC, Pegg AE. The effect of chronic ethanol administration on polyamine content during dimethylhydrazine induced colorectal carcinogenesis in the rat. Carcinogenesis 1988; 9: 2093-8. [ Links ]
34. Maeda M, Nagawa H, Maeda T, Koike H, Kasai H. Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer 1998; 83: 1483-8. [ Links ]
35. López MC, Watzl B, Hebert AR, Murphy L, Huang DS, Watson RR. Ethanol-induced changes in the intestinal mucosa-associated immune system of young and mature mice. Alcohol 1993; 10: 555-7. [ Links ]
36. Newcomb PA, Storer BE, Marcus PM. Cancer of the large bowel in women in relation to alcohol consumption: A case-control study in Wisconsin (United States). Cancer Causes Control 1993; 4: 405-11. [ Links ]
37. Riboli E, Cornee J, Macquart-Moulin G, Kaaks R, Casagrande C, Guyader M. Cancer and polyps of the colo-rectum and lifetime consumption of beer and other alcoholic beverages. Am J Epidemiol 1991; 134: 157-66. [ Links ]
38. Winkler R, Pfeiffer M, Ayisi K, Dörner A. Spontaneous colostomy cancer in rat: a handly model of colonic carcinogenesis. En: Malt RA, Williamson RCN. Colonic carcinogenesis. Boston: MTP Press Limited, Harvard Medical School, 1982. p. 245-52. [ Links ]
39. McGarrity TJ, Peiffer LP, Colony PC. Cellular proliferation in proximal and distal rat colon during 1,2.dimethylhydrazine-induced carcinogenesis. Gastroenterology 1988; 95: 343-8. [ Links ]
40. Park HS, Goodlad RA, Wright NA. The incidence of aberrant crypt foci and colonic carcinoma in dimethylhydrazine-treated rats varies in a site-specific manner and depends on tumor histology. Cancer Res 1997; 57: 4507-10. [ Links ]
41. Klurfeld DM. Dietary fiber-mediated mechanisms in carcinogenesis. Cancer Res 1992; 52 (Supl. 7): 2055s-2059s. [ Links ]
42. Hamilton SR, Sohn OS, Fiala ES. Effects of timing and quality of chronic dietary ethanol consumption on azoxymethane-induced colonic carcinogenesis and azoxymethane metabolism in Fisher 344 rats. Cancer Res 1987; 47: 4305-11. [ Links ]
43. Noguera Aguilar JF, Zurita Romero M, Tortajada Collado C, Amengual Antich I, Soro Gosálvez JA, Rial Planas R. Perianastomotic colonic tumors after inclusion of titanium or Lactomer in the anastomotic suture line. An experimental study in rats. Rev Esp Enferm Dig 2000; 92: 36-43. [ Links ]
44. Pérez-Holanda S. Réplica "Relación entre el grado de diferenciación celular del cáncer colorrectal y su distribución topográfica". Rev Esp Enferm Dig 2002, 94: 568. [ Links ]











 texto en
texto en