Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.12 Madrid dic. 2005
| ORIGINAL PAPERS |
Long-term evolution of liver histopathology in patients with chronic hepatitis C
and sustained response
M. Moreno, R. Pérez-Álvarez, L. Rodrigo, R. Pérez-López and M. González1
Departments of Gastroenterology and 1Pathology. Hospital Universitario Central de Asturias. Oviedo, Spain
ABSTRACT
Objective: to evaluate the evolution of histological changes observed in patients with chronic hepatitis C (CHC) and sustained response (SR) compared to non-responders (NR) to antiviral treatment.
Methods: a retrospective study was performed in a total of 176 patients with CHC. These were divided into two groups: 132 SR and 44 NR. All had undergone a basal liver biopsy prior to treatment onset. A second biopsy was performed in 143 patients, 104 SR and 39 NR. Inflammatory activity and the degree of liver fibrosis was measured by Metavir units (MU). The progression and regression index of fibrosis was calculated before and after treatment. The time elapsed between the two biopsies was 5.9 ± 0.3 years for SR and 6.6 ± 0.3 years for NR (NS).
Results: at baseline, 53% of SR patients had mild, chronic active hepatitis (CAH), while this was moderate in 43% of NR patients (p < 0.0001). The time elapsed between baseline and post-treatment liver biopsies was 5.9 ± 0.3 years for SR subjects and 6.6 ± 0.4 years for NR subjects (NS). After antiviral treatment 47% of SR subjects presented a normal liver or minimal changes, whilst mild CAH persisted in 34.4% and moderate CAH in 37.5% of NR subjects. Necro-inflammatory activity decreased by 50% in SR subjects and by 15% in NR subjects (p < 0.0001), and fibrosis decreased by 82% in SR subjects (p < 0.0001) and by 66% in NR subjects (p < 0.001). Fibrosis progression index was 0.14 MU/year in SR subjects and 0.21 MU/year in NR subjects (NS). Fibrosis regression index was -0.11 in SR subjects and -0.14 in NR subjects (NS).
Conclusions: in our series of patients with CHC and SR, we observed histological normalization in approximately fifty per cent of cases during long-term follow-up. NR subjects also showed improvement, especially in the fibrosis score. Both groups showed a marked regression of liver fibrosis after treatment.
Key words: Treatment of hepatitis C. Sustained response. Long-term follow-up. Liver fibrosis.
Moreno M, Pérez-Álvarez R, Rodrigo L, Pérez-López R, González M. Long-term evolution of liver histopathology in patients with chronic hepatitis C and sustained response.
Rev Esp Enferm Dig 2005; 97: 860-869.
Recibido: 19-04-05.
Aceptado: 22-06-05.
Correspondencia: Luis Rodrigo Sáez. Servicio de Aparato Digestivo. Hospital Central de Asturias. C/ Celestino Villamil, s/n. 33006 Oviedo. Fax: 985 27 36 14. e-mail: lrodrigos@terra.es
INTRODUCTION
Infection by the hepatitis C virus (HCV) is a worldwide health problem that affects more than 170 million individuals. HCV is the most prevalent type of hepatitis in the Western world. This disease presents with a marked tendency to evolve to chronicity, and this occurs in more than fifty percent. Progression to cirrhosis is frequent and occurs in 10-40% of patients, depending on the duration and the form of infection.
The progression of fibrosis in chronic hepatitis C (CHC) patients determines the need for treatment. A decrease in histological activity was shown in 50-70% of patients treated with combined treatment using either standard interferon (IFN) or pegylated interferon (PEG-IFN) combined with ribavirin. An improvement in fibrosis was observed with a minor frequency (around 20%) and was related to the type of virological response, this reaching figures as high as 90% in SR subjects and around 40% in NR subjects (1).
In a recent multicenter study carried out in a total of 153 HCV cirrhotic patients, after antiviral treatment 75 (49%) showed improved fibrosis, and cirrhosis even reverted in some cases. These results suggest that the progression of fibrosis is controlled with treatment, and even established cirrhosis may be reversible (2-7). Furthermore, the cohort analysis of studies carried out in treated patients over the long term, indicates a lower incidence of liver cancer (8-14).
A future objective will be to show whether this decrease in fibrosis may also be achieved in NR patients who have received IFN at low doses for long periods (15). Thus, two international clinical trials are currently being undertaken (EPIC-3 and HALT-C).
Here we show the results achieved by a retrospective study carried out in patients with CHC and treated with antiviral therapy, in order to evaluate histological progression in the long term.
PATIENTS AND METHODS
A total of 176 patients diagnosed with CHC, and treated consecutively with antiviral therapy in the same hospital from January 1989 to December 2003 were included.
Of these, 132 (75%) were SR and 44 (25%) NR subjects. All patients were treated with either standard IFN-alpha alone (n = 61) or in combination with ribavirin (n = 76), or pegylated IFN-alpha plus ribavirin (n = 39), and these same patients received several cycles of treatment over the study period, before obtaining a SR or being definitely classified as NR.
The time elapsed from infection to effective treatment onset, and the follow-up time from the beginning of effective treatment in SR subjects and the beginning of the initial treatment (when there was more than one) in NR subjects were evaluated.
Liver biopsies were compared pre and post-treatment. These were obtained using a tru-cut needle and abdominal ultrasonography, and all patients signed a previous informed consent. One pathologist (MG) randomly examined all samples.
One hundred and thirty one baseline liver biopsies (99%) and 107 (81%) post-treatment liver biopsies were examined in SR subjects, and 44 (100%) baseline and 39 (91%) post-treatment biopsies were examined in NR subjects. Post-treatment liver biopsies were carried out in both groups at least one year after therapy completion.
The Metavir index, which evaluates necro-inflammatory liver activity and fibrosis extent, was employed. This consists of a code system, composed of two letters and two numbers. In measuring histological activity, the interpretation is as follows: (A0 = absence; A1 = mild; A2 = moderate; A3 = severe activity), taking into account the presence of peripheral and lobular necrosis, and evaluating the presence and intensity of fibrosis: (F0 = absence; F1 = only portal, without septa; F2 = portal fibrosis with few septa; F3 = several septa, without cirrhosis; F4 = presence of cirrhosis) (16).
Progression and regression indices of liver fibrosis were calculated before and after treatment. The progression index was obtained by dividing the degree of fibrosis observed in the pre-treatment liver biopsy by the duration of infection in years. The regression index was calculated by obtaining the difference between the degree of fibrosis in pre and post-treatment liver biopsies, divided by the number of years elapsed between both.
Statistical analysis
A descriptive analysis of baseline and post-treatment characteristics of patients was made. Statistical significance was determined by means of the χ2, with Yates's continuity correction if necessary, for qualitative parameters and percentages. We compared arithmetic differences using Student's "t-test" for non-paired data, with Yates's correction, when the differences between the variances observed were statistically significant. For non-parametric data, Mann-Whitney, Wilcoxon, and Anova tests were used when necessary. A statistically significant difference between groups was considered as a p value less than 0.05.
RESULTS
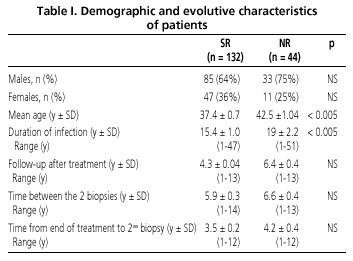
Out of a total of 176 patients, 118 (67%) were males and 58 (33%) females (male/female ratio 2/1). The distribution regarding gender, age, and other clinical and epidemiological data is shown (Table I).
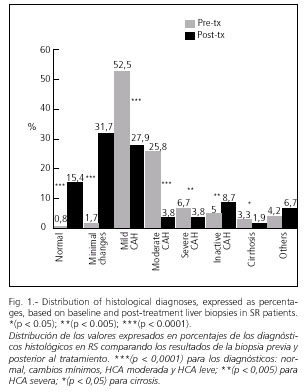
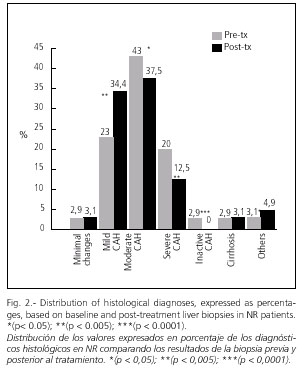
Genotype 1 was found in 69% of SR and 89% NR (p < 0.01), and genotype 3 in 24% and 5.4% respectively (p < 0.0001). A SR was achieved with PEG-IFN alfa-2b plus ribavirin in 59%, with IFN plus ribavirina in 35% and with IFN monotherapy in 6%. Histological diagnoses before and after Tx are shown in figures 1 and 2.
Among post-treatment liver biopsies, almost 50% were normal (32% with minimal changes and 15% with normal histology) in SR subjects, while the presence of moderate CAH was more prevalent in NR subjects (37%). A marked histological improvement was seen in both groups after antiviral treatment, although this was less prominent in NR compared to SR subjects (Figs. 1, 2).
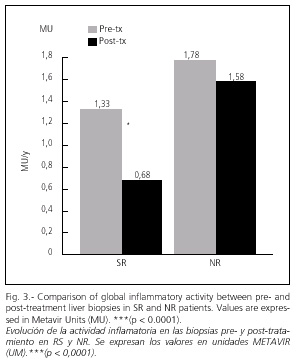
Necro-inflammatory activity notably decreased in both groups after treatment, this being 50% in SR (p < 0.0001) and 16% in NR subjects (NS) (Fig. 3).
A global histological improvement was seen in 56% of SR and 50% of NR subjects (NS). No significant liver changes were found after treatment in 38% of SR and 22% of NR subjects (p < 0.0001). A histological progression was seen in 6% of SR and 28% of NR subjects (p < 0.0001).
Liver fibrosis decreased after therapy, this being more marked in SR (50%) versus NR subjects (19%) (p < 0.0001) (Fig. 4).
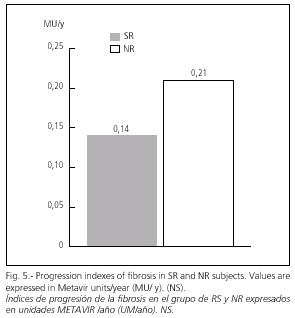
The fibrosis progression index was 0.14 ± 0.02 MU/y in SR and greater in NR subjects 0.21 ± 0.05 MU/y (NS) (Fig. 5).
The fibrosis regression index was -0.11 ± 0.03 MU/y in SR and -0.14 ± 0.05 MU/year in NR subjects (NS) (Fig. 6).
Negative values of fibrosis were obtained in both groups, this reflecting a decrease after treatment, which was more marked for NR subjects (NS). Nevertheless, when we compared regression versus progression indices, considering the latter as 100%, we observed that global fibrosis had decreased by 83% in SR (p < 0.0001) compared to 66% in NR subjects (p < 0.001) (NS between groups).
After treatment, an improvement in fibrosis was achieved in 56% of SR vs. 57% of NR subjects (NS). It remained unchanged in 29% of SR vs. 21% of NR subjects (p < 0.0005), and was worse in 15% of SR compared to 21% of NR subjects (p < 0.0005).
In the multivariate analysis performed in SR subjects, the following variables were associated with the finding of improved fibrosis in the post-treatment liver biopsy:
1. Use of combined treatment of PEG-INF plus ribavirin (p < 0.03).
2. More cycles of combined treatment (INF or PEG) (p < 0.02).
3. Higher total dose of ribavirin used (adjusted by body weight) (p < 0.05).
4. Minor degree of basal liver fibrosis (p < 0.0001).
5. Greater interval of time between both biopsies (p < 0.05).
In NR subjects improved liver fibrosis as found post-therapy was related to the following variables:
1. Lesser degree of fibrosis in the baseline biopsy (p < 0.0001).
2. Minor interval of time elapsed between biopsies (p < 0.05).
Improvement in necro-inflamatory activity was related in the SR group to:
1. Use of any combined treatment (p < 0.05).
2. Younger age at the time of treatment (p < 0.05).
3. Shorter time elapsed from the onset of infection to the beginning of effective treatment (p = 0.06).
4. Higher total dose of ribavirin (p < 0.005).
5. Minor degree of basal histological findings (p < 0.0001).
In the NR group, this was related only to the finding of a minor degree of necro-inflammatory activity before treatment (p < 0.0001).
No relationship was found for both groups between histological improvement (activity and fibrosis) and the other variables analyzed -length of infection, duration of illness, gender, viral load or genotype, duration of treatment, and total cumulative dose of INF.
DISCUSSION
Few studies exist concerning the long-term histological evolution of CHC patients who respond to antiviral treatment. Moreover, in the majority of these cases the time elapsed between both liver biopsies is relatively short (3-8 years on average), in connection with the natural duration of the illness (20 to 50 years).
For this reason, we carried out the present study in treated CHC patients, and compared the results observed in SR versus NR subjects.
Multiple studies support the predictive value of low-grade baseline fibrosis in relation to achieving a SR. The inclusion of the evaluation of four degrees of fibrosis improves the Metavir index compared to the histological activity index (HAI) proposed by Knodell (17) because of the presence of grade-2 fibrosis, and shows minor inter- and intra-observer variability (18-20).
The time elapsed from HCV infection to the beginning of treatment was similar in both groups (15 vs. 19 years). The patients received different regimens of treatment: IFN-alpha alone at the beginning, combined with ribavirin in the intermediate period, and PEG-IFN plus ribavirin in latter years. The total cumulative dose of the different drugs was greater in NR compared to SR subjects (especially for PEG-IFN alpha) (NS). The total duration of the treatment period was similar in SR and NR subjects (15 vs. 14 months). As in other studies, the best results were obtained with the combined treatment of PEG-IFN plus ribavirin, followed by the combination of standard IFN plus ribavirin, and the worst with IFN alone (21-24).
From baseline liver biopsies the most prevalent diagnosis in SR subjects was mild CAH; on the contrary, it was predominantly moderate in NR subjects. After treatment almost half of our SR patients presented with a normal liver biopsy or minimal changes. NR subjects also had histological improvement, although less markedly than SR subjects, with the number of patients with mild hepatitis increasing and the percentage of moderate and severe CAH cases decreasing. For the other diagnoses, significant differences between both groups existed except for cirrhosis, this being due to the small number of such patients included in the study.
The necro-inflammatory activity decreased in both groups with antiviral treatment, achieving a greater reduction of 50% in SR compared to only 15% in NR patients. Similarly, the degree of fibrosis decreased by 50% in SR and by 19% in NR subjects (p < 0.0001).
Although the main objective of antiviral therapy in CHC is the eradication of HCV, it is possible that treatment could improve the natural history of disease in NR patients, without achieving viral eradication.
Numerous studies have shown that interferon therapy decreases the severity of lobular and portal inflammation in NR patients (3,25,26), and also a 50% reduction in the inflammatory component of the Knodell index has been observed at end of treatment (EOT) in around 75% of patients who had a biochemical response, and in 38% of NR subjects (27). This has been confirmed in a recent meta-analysis that clearly demonstrates a histological improvement in 51% of treated patients, in spite of not achieving a normalization of liver function tests (28).
Because fibrosis progression is mediated by inflammation, its improvement or disappearance may achieve a reduction of long-term fibrosis. This effect is more obvious in patients who have a sustained biochemical and virological response (29-31), but may also occur in NR patients (32) as has been observed in our study.
For this reason, and in accordance with our results and the findings reported by other authors, we may conclude that the anti-fibrotic effect of interferon is not only limited to SR subjects, but occurs in the majority of treated patients, including those who are NR (33,34).
These findings lead us to suggest that repeated cycles of treatment may slow the spontaneous progression of liver fibrosis or even allow its regression even in the presence of cirrhosis. This antifibrogenic effect should be always considered in the strategies of treatment for new and older CHC patients, and in the analysis of combined therapy using pegylated interferon with other antiviral drugs (35).
We found that the fibrosis progression index was (0.14 MU/y) in SR subjects, this being similar to values found by several authors (36-40), and slightly higher in NR subjects (0.21 MU/y), but without statistical differences between both groups (NS).
After treatment, the fibrosis regression index decreased to -0.11 MU/y in SR and to -0.14 MU/y in NR subjects (NS). Expressed in another terms, the percentage decrease was 82% in SR and 66% in NR subjects.
In other previous studies fibrosis decreased after treatment by 55% both in SR and NR subjects. It worsened in 13% of SR compared to 22% of NR individuals, and remained without change in 31% of SR and in 22% of NR individuals (2,3).
We can conclude that NR patients develop fibrosis quicker than SR patients, and it can return and even disappear after antiviral treatment. Our results confirm that NR can also improve fibrosis, and this implies important clinical and therapeutic consequences.
Factors related with the improvement of fibrosis after treatment in SR subjects were the use of combined treatment with PEG-IFN and ribavirin, a greater number of cycles of combined treatment, a higher total dose of ribavirin, a lower baseline degree of fibrosis, and a longer time interval between both biopsies as being important. In NR subjects we only found a relationship with a lower degree of baseline fibrosis and a shorter interval between biopsies, but it must be born in mind that the sample was not large enough.
Regarding factors implicated in the improvement of activity in SR subjects, we found that the use of combined treatment, a younger age at the beginning of treatment, a shorter evolution of infection, a greater total dose of ribavirin, and a minor degree of baseline liver damage are of prognostic significance. However, in NR patients this improvement was only found in patients who presented with a minor necro-inflammatory baseline activity.
The finding of a relationship with higher doses of ribavirin strongly supports the theory of dose-dependent anti-inflammatory activity that synergistically influences the anti-fibrogenic and anti-viral effect of IFN in SR subjects.
In a previous study, all types of treatment used improved the fibrosis regression index after treatment. The following six factors were found to be related with better response: lower degree of basal fibrosis, presence of SR, younger age (less than 40), BMI < 27 kg/m2 , minimal inflammatory activity, and viral load lower than 3.5 million copies/ml (2).
Given the slow recovery of the fibrotic process in NR CHC patients, long-term treatment with PEG-IFNa alone or combined with ribavirin in low doses may be a therapeutic option to slow down liver fibrosis. However, before making this recommendation, we must wait for the results of ongoing clinical trials.
ACKNOWLEDGEMENTS
We wish to thank Dr. Enrique Bustillo for his help in the statistical analysis study, and Doctors Antonio Linares, Nieves González, and María-Isabel Martínez for their cooperation in the performance of hepatic biopsies. We also wish to thank David H. Wallace (member of the Council of Scientific Editors and the European Association of Scientific Editors) for his critical revision of the manuscript.
REFERENCES
1. Manns M, Mc Hutchison JC, Gorgon S, et al. Peginterferon alfa-2b plus ribavirin compared to interferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C; results of a randomized trial. Lancet 2001; 358: 958-65. [ Links ]
2. Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa 2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002; 122: 1303-13. [ Links ]
3. Stanislas P, Françoise C, Bertrand N, et al. Reversibility of hepatitis C virus-related cirrhosis. Human Pathology 2004; 35: 107-12. [ Links ]
4. Lindsay K, Trepo C, Heintges T, et al. A randomised, double blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 2001; 34: 395-403. [ Links ]
5. Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med 2000; 343: 1673-80. [ Links ]
6. Heathcote EJ, Shiffman ML, Cooksley WGE, et al. Peginterferon alfa-2a in patients with chronic hepatitis c and cirrhosis. N Engl J Med 2000; 343: 1666-72. [ Links ]
7. Beyond RC, Iredale JC. Is liver fibrosis reversible? Gut, 2000; 46: 443-6. [ Links ]
8. Baffis V, Shrier I, Sherker AH, Szilagyi A. Use of interferon for prevention of hepatocellular carcinoma in cirrhotic patients with hepatitis B or hepatitis C virus infection. Ann Intern Med 1999; 131: 696-701. [ Links ]
9. Hayashi K, Kumada T, Nakano S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis C after interferon therapy. Hepatogastroenterology 2002; 49: 508-12. [ Links ]
10. Hino K, Kitase A, Satoh Y, et al. Interferon retreatment reduces or delays the incidence of hepatocellular carcinoma in patients with chronic hepatitis C. J Viral Hepatol 2002; 9: 370-6. [ Links ]
11. Imazeki F, Yokosuka O, Fukai K, et al. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology 2003; 38: 493-502. [ Links ]
12. Pol S, Carnot F, Nalpas B, et al. Reversibility of hepatitis C virus-related cirrhosis. Hum Pathol 2004; 35: 107-12. [ Links ]
13. Shiffman M, Hofman CM, Thompson EB, et al. Relationship between biochemical, virological, and histological response during interferon treatment of chronic hepatitis C. Hepatology 1997; 26: 780-5. [ Links ]
14. Alri L, duffaut M, Selves J, et al. Maintenance therapy with gradual reduction of the interferon dose over one year improves histological response in patients with chronic hepatitis C with biochemical response: results of a randomized trial. J Hepatol 2001; 35: 272-8. [ Links ]
15. Beddosa P, Poynard T. The METAVIR cooperative study group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24: 289-93. [ Links ]
16. Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numeral scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981; 1: 431-5. [ Links ]
17. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20: 15-20. [ Links ]
18. Theodossi A, Skene AM, Portmann B, et al. Observer variation in assessment of liver biopsies including analysis by Kappa statistics. Gastroenterology 1980; 79: 232-41. [ Links ]
19. Beddosa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38: 1449-57. [ Links ]
20. Fried M, Shiffman M, Reddy R, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975-82. [ Links ]
21. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon a-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon a-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998; 352: 1426-32. [ Links ]
22. McHutchinson JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998; 339: 1485-92. [ Links ]
23. Davis GL, Esteban-Mur R, Rustgi VK, et al; Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med 1998; 339: 1493-9. [ Links ]
24. Wu J, Danielsson A. Inhibition of hepatic fibrogenesis: a review of pharmacologic candidates. Scand J Gastroenterol 1994; 29: 385-91. [ Links ]
25. Brenner DA, Alcorn JM. Therapy of hepatic fibrosis. Semin Liver Dis 1990; 10: 75-83. [ Links ]
26. Shiffman M. Retreatment of VHC non-responders with peginterferon and ribavirin: results fron the lead in phase of the hepatitis C antiviral long term treatment against cirrosis (HALT-C) trial. Hepatology 2002; 36: 295 A. [ Links ]
27. Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000; 132: 517-24. [ Links ]
28. Camps J, Castilla A, Ruiz J, et al. Randomized trial of lymphoblastoid a-interferon in chronic hepatitis C. Effects on inflammation, fibrogenesis and viremia. J Hepatol 1993; 17: 390-6. [ Links ]
29. Guido M, Rugge M, Chemello L, et al. Liver stellate cells in chronic viral hepatitis: the effect of interferon therapy. J Hepatol 1996; 24: 301-7. [ Links ]
30. Moreno MG, Muriel P. Remission of liver fibrosis by interferon-a2b. Biochem Pharmacol 1995; 50: 515-20. [ Links ]
31. Cammà C, Giunta M, Linea C, Pagliaro L. The effect of interferon on the liver in chronic hepatitis C: a quantitative evaluation of histology by meta-analysis. J Hepatol 1997; 26: 1187-99. [ Links ]
32. Sobesky R, Mathurin P, Charlotte F, et al. For the MULTIVIRC group. Modeling the impact of alpha interferon treatment on liver fibrosis progression in chronic hepatitis C; a dynamic view. Gastroenterology 1999; 116: 378-86. [ Links ]
33. Poynard T, Moussali J, Ratziu V, Regimbeau C, Opolon P. Effects of interferon therapy in "non responder" patients with chronic hepatitis C. J Hepatol 1999; 31: (Supl. 1): 178-83. [ Links ]
34. Davis Gl, Schiff E, Marcellin P, Esteban Mur R, Goodman Z, et al. Long-term continuous recombinant interferon alfa-2b (Intron A) versus repeated 24 week cycles for disease suppression in IFN relapsers with chronic hepatitis C. Hepatology 1999; 30 (Supl. A): 317A. [ Links ]
35. Poynard T, Bedossa P, Opolon P, for the OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC Groups. Natural History of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997; 349: 825-32. [ Links ]
36. Poynard T, Ratziu V, Charlotte H, et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol 2001; 34: 730-9. [ Links ]
37. Marcellin P, Akrémi R, Cazals D, et al. Genotype 1 is associated with a slower progression of fibrosis in untreated patients with mild chronic hepatitis C. Hepatology 2001; 34 (Supl. 1): 159 A. [ Links ]
38. Ghany MG, Keiner DE, Alter HJ, et al. Progression of fibrosis in early stages of chronic hepatitis C. Hepatology 2000; 32: 496 A. [ Links ]
39. Alberti A, Boccato S, Ferrari A, et al. Outcome of initially mild chronic hepatitis C. Hepatology 2001; 34: 225 A. [ Links ]











 texto en
texto en