Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.98 no.8 Madrid ago. 2006
ORIGINAL PAPERS
MRI and endoscopic ultrasonography in the staging of gastric cancer
Resonancia magnética y ecoendoscopia para el estadiaje del cáncer gástrico
M. G. Arocena, A. Barturen1, L. Bujanda2, O. Casado3, M. M. Ramírez4,
J. M. Oleagoitia1, M. Galdiz Iturri, P. Múgica, A. Cosme2,
M. A. Gutiérrez-Stampa2, E. Zapata and M. Echenique-Elizondo5
Department of Surgery. San Eloy Hospital. Baracaldo, Vizcaya. Spain.
1Department of Gastroenterology. Cruces Hospital. Baracaldo, Vizcaya. Spain.
2Department of Gastroenterology. Donostia Hospital. San Sebastián, Guipúzkoa. Spain.
3Department of Radiology. Service of Radiology. OSATEK. Spain.
4Department of Pathology. San Eloy Hospital. Baracaldo, Vizcaya. Spain.
5Department of Surgery. Basque Country University. Spain
ABSTRACT
Objective: to determine the diagnostic precision of endoscopic ultrasounds (EUS) and magnetic resonance imaging (MRI) in the preoperative staging of gastric cancer.
Methods: a prospective, blind study was carried out in 17 patients diagnosed with gastric cancer (GC) using endoscopic biopsy from November 2002 to June 2003. Patients underwent preoperative MRI and EUS. The reference test used was pathology, and laparotomy for non-resectable cases.
Results: MRI (53%) was better than EUS in the assessment of gastric wall infiltration (35%). MRI (50%) was also superior to EUS (42%) for N staging. After pooling stages T1-T2 and T3-T4 together, results improved for both MRI (67 and 87.5%, respectively) and EUS (67 and 62.5%, respectively) (p < 0.05). N staging -lymph node invasion- results were correct in 50% for MRI as compared to EUS (42%). In classifying positive and negative lymph nodes EUS was superior to MRI (73 versus 54%).
Conclusions: MRI was the best method in the assessment of gastric wall infiltration. EUS was superior to MRI for T1 staging, and in the assessment of lymph node infiltration.
Key words: Magnetic resonance imaging. Endoscopic ultrasound. Gastric cancer.
Introduction
Gastric carcinoma is one of the commonest gastrointestinal tumors. The prognosis and survival rate for gastric cancer (GC) is poor in advanced stages (1) (Table I); 44% of patients with stage T2 and 64% of patients with T3 show lymph node infiltration (2). Surgery is the only curative treatment, but few patients are resectable at the time of diagnosis. Moreover, GC surgery has a high morbidity rate, and that is why accurate tumor staging is so important before surgery.
Currently available among the techniques for preoperative imaging in GC staging is helical computerized tomography (CT). The accuracy of CT to disclose wall infiltration varies between 25 and 66%, lymph node infiltration between 25 and 68%, and metastatic disease identification between 65 and 72% (3). That is the reason why other diagnostic techniques must be included to improve results in GC staging.
In recent years, magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS) have been used for tumor staging evaluation. MRI has offered similar or even better results in tumor staging (4,5). EUS has provided a new dimension for local invasiveness assessment in esophageal (6) and rectal tumors (7). Some authors demonstrated that EUS is an adequate procedure with great diagnostic accuracy in establishing degree of infiltration of the gastric wall and lymph nodes in GC (8-10).
Our aim was to evaluate the accuracy of EUS and MRI in the preoperative staging of gastric cancer.
Material and methods
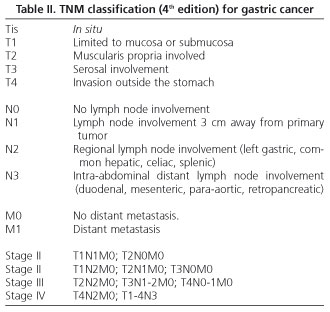
A prospective, blind study was performed to evaluate the accuracy of EUS and MRI in the preoperative staging of gastric cancer. It included patients (n = 21) diagnosed with GC by endoscopic biopsy from November 2002 to June 2003 in "San Eloy" Hospital. The gold standard test used for staging was pathological findings in resected cases and laparotomy in non-resectable cases. Exclusion criteria included: patients with pacemakers, claustrophobia, tumors with stenoses that prevented endoscopic progress, patients with liver and/or lung metastases, a delay of more than 7 days between the different diagnostic techniques, and a delay of more than 20 days between the endoscopic diagnosis and surgery. GC staging was carried out using the TNM classification -4th edition (11) (Table II).
Out of the 21 patients included in the study, 19 underwent surgery.
Surgery was done by two surgeons at the Esophageal-Gastric Unit. Pathological examination was performed by an expert pathologist on gastric lesions. The Hospital's Ethical Committee approved this study. A signed consent form was obtained from each patient.
All MRI studies were performed by the same radiologist with wide experience in gastric disease examination. A 1T magnet with a coil paced array was employed. Patients were fasted and positioned in supine after taking 400 ml of diluted oral gadolinium (1/10 dilution). No muscle relaxants were administered. In-phase and out-of-phase T1 GE sequences were performed (TR:135, TE:3.7, slice thickness: 7 mm) with breath holds on at least two sections, always including an axial section from the gastro-esophageal union to the lower kidney pole; HASTE T2 (TR:1100, TE:120, slice thickness: 5 mm) with breath holds on all three sections, limited to the stomach, T1 (TR:587, TE:12, slice thickness: 8 mm) and STIR (TR:5000, TE:60, TI:130, slice thickness: 7-8 mm) axials from the liver dome to below the kidneys. When required, oblique coronal and sagittal sections of the tumor were taken in T1 GE sequence, in-phase and out-of-phase. Finally, an in-phase and out-of-phase GE T1 study was performed axially with dynamic breath hold using intravenous gadolinium (TR:135, TE:3.7, thickness: 7 mm). Nodes > 10 mm were considered positive.
EUS was performed by one experienced endoscopist. EUS involved the use of an EUM20 oblique echo-endoscope (Pentax FG-36UX). All patients were also studied by means of a 12.5 MHz probe. Lymph nodes were considered positive if larger than 5 mm, round, hypoechoic, well demarcated, and homogenous (12). No FNA was performed in association with EUS.
Statistical analysis
Sensitivity, specificity, predictive positive value (PPV), predictive negative value (PNV), and diagnostic accuracy for both MRI and EUS, and T and N stage was carried out by a contingence tetraconic (2 x 2) analysis. To avoid mathematic artefacts a binomial exact test -Miettinen's or Fleiss'- was used. A Wilcoxon's signed rank test was used to demonstrate differences. Results were considered significant when p < 0.05. Percentile-expressed values that were not significant included 50% of CI or 1 for PP quotients and PN quotients.
Results
Nineteen of all 21 patients included were operated upon. Surgery was not performed on 2 patients -in one case because the patient was elderly and had comorbidities; in the other case because the patient had carcinomatous ascites as confirmed by cytology following puncture. Of all 19 patients who underwent surgery, 5 cases were non-resectable due to extensive local infiltration and/or carcinomatosis, and the adherent lymph nodes in cumuli. All 19 patients underwent MRI studies, and 17 underwent MRI and EUS examinations. EUS could not be performed in 2 patients because they would not meet protocol requirements: one had a delay of more than 7 days; the other had an almost total stenosis due to an antro-pyloric tumor, which would have prevented a correct assessment.
Of all 19 operated patients 14 were men and 5 were women. Average age was 70 (ranging from 56 to 81 years). The tumor was antral in 12 cases (63%), corporal in 3 cases (15%), and located in the upper third of the stomach in 4 cases (21%). Histological findings were: intestinal adenocarcinoma in 11 patients and diffused adenocarcinoma in 8 cases (all non-resectable cases were in this group).
Surgical procedures performed included: gastrectomy, omentectomy and D1-D2 lymphadenectomy. A total gastrectomy was performed in 5 patients, and 9 patients underwent subtotal gastrectomy with Billroth I, II or III reconstructions, depending on regional conditions and surgeon preferences. Isolated lymph nodes varied from 8 to 29 (average 15).
There was no postoperative mortality; 5 patients (35%) presented with postoperative complications -3 low flow fistulas, one lymphorrhagia, and a sub-hepatic abscess. All complications resolved with medical treatment except the abscess, which required surgical drainage.
Final tumor staging according to the gold standard was: one patient at stage T1, 7 patients at T2, 3 at T3, and 6 at T4. With regard to N staging, 6 patients were at stage N0, 4 were at N1, 1 was at N2, and 6 were classified at N3. No patients presented metastases (M0).
For T staging, MRI accurately assessed 53% of cases, and understaged or overstaged 23.5% of cases. EUS accurately assessed 35% of cases, understaged 47%, and overstaged 18% of cases. Sensitivity for T4 staging by EUS was 20%.
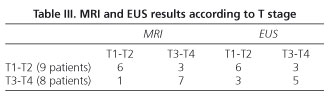
When studied by grouping T1 and T2, and T3 and T4 together, the following results were obtained: MRI accurately assessed 67% of cases in the T1-T2 group and 87.5% in the T3-T4 group, while EUS accurately assessed 67% of cases in the T1-T2 group and 62.5% in the T3-T4 group (Table III). There was only one T1 patient who was diagnosed by EUS and overstaged as T3 by MRI.
For N staging, MRI accurately assessed 50% of cases, understaged 25%, and over-staged 25% of cases. EUS accurately assessed 42% of cases, and also understaged 42% and overstaged 16% of cases. Sensitivity for N0 in MRI was 50 versus 67% in EUS. When analyzing N1, N2 or N3 as positive, MRI correctly predicted 60% of cases, and EUS 70.5% of cases (Table IV). There were no significant differences between both diagnostic techniques.
Table V shows the sensitivity, specificity, positive predictive value, and negative predictive value of each technique.
Discussion
Preoperative imaging techniques for gastric cancer staging sometimes fail, leading to unnecessary surgery because of tumor non-resectability (2). Nowadays two techniques -MRI and EUS- are employed for GC staging. Some ex vivo studies have demonstrated that MRI has a diagnostic accuracy of almost 100% for gastric wall invasion by the tumor (13). In this study MRI diagnostic accuracy was 76% for T assessment, similar to figures obtained in other studies and to those encountered for CT scans (Table VI).
EUS is the gold-standard technique for the T staging of esophageal (6) and rectal (7) cancer. EUS has not been widely assessed in GC staging. In our study, T staging with EUS -when stages T1 and T2, and T3 and T4 were grouped together- was low: a 65% lower than results obtained for esophageal cancer (6). This could be because only one case with stage T1 was accurately diagnosed with EUS, and distinguishing this from peritumoral inflammatory events is difficult.
The correct staging of node involvement is very important, as it involves more aggressive surgery and more aggressive chemo- and radio-therapy. For the majority of authors, the diagnostic accuracy of helical CT is less than 75% (4,5,20,21). When CT and MRI are compared in N staging, results are similar (Table VI). In our study MRI showed a low diagnostic accuracy of 53%, similar to that found in EUS. One possible explanation for these poor results for lymph node invasion is the lack of consensus regarding the criteria to assess malignant-looking lymph nodes, since some studies consider positive nodes that are 5 mm in size, whereas others considerer positive nodes larger than 8 or 10 mm. Other confusing factors can be inflammatory changes in nodes or small tumor-invasion areas without node enlargement. MRI does not accurately evaluate lymph nodes that are close to the gastric wall, while EUS evaluates more properly nodes far removed from the stomach.
Other techniques, including tridimensional CT scanning, hydro-CT, and positron-emission tomography (PET), offer good results in the preoperative staging of gastric cancer (22-24).
In spite of considering the small number of patients studied, it is our opinion that EUS must be used when MRI or CT show a T1 or T2 stage in the absence of distal metastatic disease. Further studies are required with more patients in order to assess the findings observed in this study.
Acknowledgements
We thank S. Álvarez Ruiz, M.D., Nuclear Medicine Department, Santiago Hospital (Vitoria, Spain) for his critical reading and statistics.
References
1. Koh TJ, Wang TC. Tumors of the stomach. En: Feldman M, Friedman LS, Slesinger MH, editors. Gastrointestinal and liver diseases. 7ª ed. Philadelphia: Saunders; 2002. p. 829-48. [ Links ]
2. Hohenberger P, Gretschel S. Gastric cancer. Lancet 2003; 362: 305-15. [ Links ]
3. Fukuya T, Honda H, Haneko K, Kmoiwa T, Yoshimitsu K, Irie E, et al. Efficacy of helical CT in T staging of gastric cancer. J Comput Assist Tomogr 1997; 21: 73-81. [ Links ]
4. Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR 2000; 174: 1551-7. [ Links ]
5. Kim AY, Han JK, Seong CK, Kim TK, Choi BI. MRI in staging advanced gastric cancer: Is it useful compared with spiral CT? J Comput Assist Tomogr 2000; 24: 389-94. [ Links ]
6. Penman ID, Shen EF. EUS in advanced esophageal cancer. Gastrointest Endosc 2002; 56 (Supl. 4): 2-6. [ Links ]
7. Savides TJ, Master SS. EUS in rectal cancer. Gastrointest Endosc 2002; 56 (Supl. 4): 12-8. [ Links ]
8. Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, et al. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology 1991; 181: 426-32. [ Links ]
9. Lightdale CJ. Endoscopic ultrasonography in the diagnosis, staging and follow-up of esophageal and gastric cancer. Endoscopy 1992; 24: 297-303. [ Links ]
10. Ziegler K, Sanft C, Zimmer T, Zeitz M, Felsenberg D, Stein H, et al. Comparison of computed tomography, endosonography, and intraoprative assessment in TN staging of gastric carcinoma. Gut 1993; 34: 604-10. [ Links ]
11. Hermanek P, Sobin LH. Digestive system tumors. In: Hermanek P, Sobin LH, eds. TNM classification of malignant tumors. 4th ed, 2nd rev. New York: Springer-Verlag, 1992. p. 45-8. [ Links ]
12. Vickers J, Alderson D. Oesophageal cancer staging using endoscopic ultrasonography. Br J Surg 1998; 85: 994-8.10. [ Links ]
13. Sato C, Naganawa S, Kumada H, Miura S, Ishigaki T. MR imaging of gastric cancer in vitro: accuracy of invasion depth diagnosis. Eur Radiol 2004; 14: 1543-9. [ Links ]
14. Grimm H, Soehendra N, Hamper K, Maas R. Contribution of endosonography to preoperative staging in esophageal and stomach cancer. Chirurg 1989; 60: 684-9. [ Links ]
15. Natterman C, Galbenu-Grunwald R, Nier H, Dancygier H. Endoscopic ultrasound in TN staging of stomach cancer. A comparison with computerized tomography and conventional ultrasound. Z Gesamte Inn Med 1993; 48: 60-4. [ Links ]
16. Isozaki H, Okajima K, Nomura E, Fujii K, Sako S, Izumi N, et al. Preoperative diagnosis and surgical treatment for lymph node metastasis in gastric cancer. Gan To Kagaku Ryoho 1996; 23: 1275-83. [ Links ]
17. Perng DS, Jan CM, Wang WM, Chen LT, Su YC, Liu GC, et al. Computed tomography, endoscopic ultrasonography and intraoperative assessment in TN staging of gastric carcinoma. J Formos Med Assoc 1996; 95: 378-85. [ Links ]
18. Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska-Guttmejer A, Butruk E. Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy 2004; 36: 617-23. [ Links ]
19. Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc 2004; 59: 619-26. [ Links ]
20. Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, et al. Lymph-node metastases: efficacy of detection with helical CT in patients with gastric cancer. Radiology 1995; 197: 505-11. [ Links ]
21. Adachi Y, Sakino I, Matsumata T, Iso Y, Yoh R, Kitano S, et al. Preoperative assessment of advanced gastric carcinoma using computed tomography. Am J Gastroenterol 1997; 92: 872-5. [ Links ]
22. Yun M, Lim JS, Hoh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18) F-FDG PET: a comparison study with CT. J Nucl Med 2005, 46: 1582-8. [ Links ]
23. Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging 2005; 30: 465-72. [ Links ]
24. D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol 2000; 10: 1877-85. [ Links ]
![]() Correspondence:
Correspondence:
Luís Bujanda.
Avda. Sancho El Sabio 21, 3º C.
20010 San Sebastián. Guipúzcoa.
Fax: 943 006 407. E-mail: castro@medynet.com
Recibido: 14-07-05
Aceptado: 22-02-06











 texto en
texto en