Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.99 no.10 Madrid oct. 2007
CLINICAL NOTE
Acute GI bleeding by multiple jejunal gastrointestinal autonomic nerve tumour associated with neurofibromatosis type I
Urgencia quirúrgica por sangrado intestinal debido a tumor intestinal de nervios autónomos asociados a neurofibromatosis tipo I
M. Keese, T. Riester, K. Schwenke, D. Dinter1, W. Back2 and P. Palma
Departments of Surgery, 1Radiology and 2Pathology. University Hospital of Mannheim. Germany
RESUMEN
Se describe una urgencia quirúrgica por sangrado intestinal debido a tumor gastrointestinal de nervios autónomos (GANT) asociado a enfermedad de von Recklinghausen.
Una mujer de 72 años con neurofibromatosis fue ingresada con signos de melena. La endoscopia digestiva alta y baja fue negativa. Se indicó TAC con contraste que advirtió tumores yeyunales como causa del sangrado. Se realizó laparotomía y resección de un segmento de 20 cm de yeyuno que incluía varios tumores.
La causa del sangrado activo fue lesión en mucosa intestinal por erosión tumoral. El análisis por inmunohistoquímica de la pieza mostró diferenciación neuronal, con células fusiformes con tinción positiva para el C-Kit (CD 117), CD 34.
Esta nota clínica pone de manifiesto la asociación entre la neurofibromatosis y los tumores estromales y debe alertar a gastroenterólogos y cirujanos sobre las posibles manifestaciones intestinales en pacientes con enfermedad de von Recklinghausen.
Palabras clave: Tumor gastrointestinal de nervios autónomos. Enfermedad de von Recklinghausen. Tumores estromales. Sangrado gastrointestinal.
ABSTRACT
We describe a surgical emergency due to GI-bleeding caused by gastrointestinal autonomic nerve tumours (GANT's) in a patient with von Recklinghausen's disease.
A 72 year old female patient with von Recklinghausen's disease was admitted with maelena. Endoscopy showed no active bleeding in the stomach and the colon. Therefore an angio-CT-scan was performed which revealed masses of the proximal jejunum as source of bleeding. Laparotomy was indicated and a 20 cm segment of jejunum which carried multiple extraluminal tumours was resected.
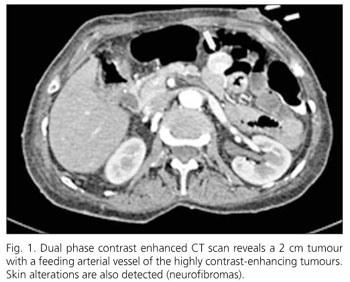
The source of the bleeding was a 2 cm tumour which had eroded the mucosal surface. Immunohistologically, evidence of neuronal differentiation could be shown in the spindle-formed cells with positive staining for C-Kit (CD 117), CD 34, and a locally positive staining for synaptophysine and S100.
This case report illustrates the association between neurofibromatosis and stromal tumours and should alert surgeons and gastroenterologist about gastrointestinal manifestations in patients with von Recklinghausen's disease.
Key words: Gastrointestinal autonomic nerve tumours. von Recklinghausen's disease. Stromal tumors. Gastrointestinal bleeding.
Introduction
Neurofibromatosis type I is an autosomal dominant disorder with variable penetrance. The major diagnostic criteria include skin lesions such as café-au-lait spots, axillary and inguinal frecklings, multiple neurofibromas, Lisch nodules (pigmented iris hamartomas), optical gliomas and bone lesions and a first degree family history of NF (1). The responsible gene for NF1 codes for a GTPase-activating protein (neurofibromin), which regulates the activity of of the protooncogene p21 ras. Therefore neurofibromatosis patients are at an increased risk to develop malignant tumours (2).
Gastrointestinal (GI) involvement in von Recklingshausen's disease occurs in three principal forms: hyperplasia of the submucosal and myenteric nerve plexuses and mucosal ganglioneuromatosis lead to disordered gut motility, gastrointestinal stromal tumours (GISTs) showing varying degrees of neuronal or smooth muscle differentiation and carcinoids of the periampullary region of the duodenum (3). The identification of gastrointestinal stromal tumours which arise from autonomic nerves in the bowel wall (gastrointestinal autonomic nerve tumours, GANT's), originally described as plexosarcomas, is based on ultrastructural similarities with the myenteric plexus of the gastrointestinal tract (3,4). These tumours represent a distinct subcategory of gastrointestinal stromal tumours. There is also increasing evidence in the literature for the association of von Recklinhausen's disease and GANT's of the small bowel (5,6). Here we describe a surgical emergency due to GI-bleeding caused by GANT's in a patient with von Recklinghausen's disease.
Case report
A 72-year old female patient presented with a history of maelena. Von Recklingshausen's disease was diagnosed 30 years ago. Physical examination showed a clearly reduced nutritional status and the typical café au lait spots as well as multiple skin neurofibromas. During physical assessment blood was found upon digital examination of the rectum. To exclude a bleeding source in the stomach or duodenum a gastroscopy was performed which showed no abnormalities. At colonoscopy blood was found in the terminal ileum, however, the active bleeding source could not be identified. Because GI bleeding persisted, two units of whole banked blood were transfused over the first two days. A dual-phase angio-CT-scan (Somatom Volume Zoom, Siemens, Erlangen, Germany) after i.v. application of 120 ml iodinized contrastmedia was performed, which localized a bleeding focus in extraluminal tumours of the proximal jejunum (Fig. 1).
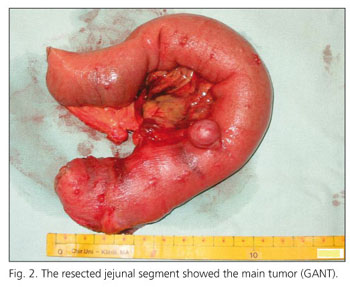
After laparotomy, multiple extraluminal tumours were found on the serosal side of the proximal jejunum (Fig. 2). The proximal resection margin was chosen directly after Treitz ligament to allow a 2 cm distance to the most proximal tumour and a 20 cm long segment was resected. On the opened specimen, one larger tumour had eroded into the mucosal surface. Bowel continuity was restored by means of a retrocolical latero-terminal duodeno-jejunostomie.
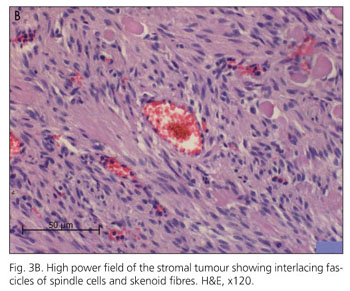
Pathological examination of the resected jejunum showed multiple small tumour nodules on the serosa in close proximity to several larger maximally 2.5 cm tumours. These larger tumours infiltrated all layers of the bowel wall and some eroded the mucosa. Tumours consisted of uniform spindle-formed cells with an eosinophilic cytoplasm (Fig. 3A). Locally a hyperplasia of the plexus myentericus could be found. Smaller tumours in the serosa and subserosa showed a clearly positive staining for CD 34, NSE and S100 (Fig. 3B). No staining could be found for neurofilaments and SMA. Larger tumours with infiltration of the mucosa also stained strongly positive for C-Kit (CD 117) and CD 34. These are typical features of gastrointestinal stromal tumours of autonomous nerve origin.
The postoperative period was uneventful. The patient was advised to attend a regular follow up. In the three year follow up, no recurrence could be detected.
Discussion
Neurofibromatosis is one of the most frequent hereditary diseases. While also several rare mosaic forms are described neurofibromatosis 1 is the commonest form with up to 20 times as frequent as NF2 (7). Neurofibromatosis 1 and 2 are autosomal dominant diseases with half of the cases being attributed to new mutations. There is an increased risk to develop malignant tumours in NF1. The responsible gene which has been localized to chromosome 17 (gene locus 17q11.2) codes for a GTP-ase which is expressed in all cell types at low amounts, in high amounts it can be found in neuroectodermal cells (8,9). Physiologically, neurofibromin 1 binds with the gap120-like to p21 ras which leads to inactivation and consequentially to cellular differentiation and to an inhibition of cell growth. This inhibitory function is lost with a mutated and defective neurofibromin gene product (9).
The identification of GANT's, originally described under the name plexosarcomas has been based on ultrastructural similarities with the myenteric plexus of the gastrointestinal tract (10). Ultrastructurally, the presence of dense core granules, cell processes, neurotubules, dystrophic axons, synapse-like structures and skenoid fibers are described to be characteristic for these tumours (11,12). Gain of function c-KIT-mutations were found in extracellular, juxtamembrane and kinase domains presumably contributing to the molecular initiation of these neoplasms (5).
GANT's normally tend to be semimalignant. Nevertheless complete en bloc surgical resection, when possible, is the cornerstone of their therapy (13). A more radical resection in the present case would have been a pylorus preserving pancreaticoduodenectomy since also metastatic disease and tumour recurrence have been described in patients with GANT's. In our patient, however, the tumours had manifested themselves by severe GI-bleeding and therefore a less radical approach was pursued, especially since liver metastases or intraperitoneal spread are a rare phenomenon and tend to occur mainly in patients with tumours that have ruptured spontaneously or which have been violated by the surgeon.
Gastrointestinal tumour manifestation in neurofibromatosis has been described and typically varies from gut neuronal tissue hyperplasia to gastrointestinal stromal and endocrine tumours. Also other neoplasms can be found (14) but this is generally considered a rare event. GANT's are a rare but distinct subcategory of gastrointestinal stromal tumours. In the majority of the cases which have been described so far, the primary site of the tumour was the stomach, duodenum, jejunum or ileum, but also oesophagus, rectum, bladder or colon have been reported (6). Stromal tumours remain mainly asymptomatic until the tumour reach a sufficient size to produce abdominal pain or mucosal ulceration with GI-bleeding as reported in our case. At this point, the clinical symptoms depend on the site of the manifestation. Symptoms could also be related to the luminal obstruction but this is a late event due to the serosal origin of the tumour (15). GI-bleeding as a consequence of erosion of the mucosa by GIST has so far only been reported in one patient with neurofibromatosis (16). This report should alert surgeons and gastroenterologists to the rare GI-tract manifestations of tumours in von Recklingshausen's disease. In as much as GANT's have to be treated by surgery and especially in the case of GI-bleeding as reported here, we propose a dual phase angio-CT as first cornerstone of the diagnostic of neurofibromatosis patients with any gastrointestinal symptoms.
References
1. Riccardi VM. The clinical and molecular genetics of neurofibromatosis-1 and neurofibromatosis-2. In: Rosenberg RN, Prusiner SB, DiMauro S, Bachi RL, Kunkel LM, editors. The molecular and genetic basis of neurological disease. Boston: Butterworth-Heinemann; 1993. p. 837-53. [ Links ]
2. McCormick F. Ras signaling and NF1. Curr Opinion Genes Dev 1995; 5: 51-5. [ Links ]
3. Miettinen M, Fetsch JF, Sobin LH, Lasota JL. Gastrointestinal stromal tumours in patients with neurofibromatosis 1 A. clinicopathologic and molecular genetic study of 45 cases. Am J Pathol 2006; 30: 90-6. [ Links ]
4. Herrera GA, Pinto de Morales H, Grizzle WE, Han SG. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma). Light and electron microscopic study confirming the origin of the neoplasm. Dig Dis Sci 1984; 29: 275-84. [ Links ]
5. Debiec-Rychter M, Pauwels P, Lasota J, Franke S, De Vos R, de Wever I, et al. complex genetic alterations in gastrointestinal stromal tumours with autonomic nerve differentiation. Mod Pathol 2002; 15: 692-8. [ Links ]
6. Stift A, Friedl J, Gnant M, Herbst F, Jakesz R, Wenzl E. Gastrointestinal autonomic nerve tumours: A surgical point of view. World J Gastroenterol 2004; 10: 2447-51. [ Links ]
7. Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology 2001; 56: 1433-43. [ Links ]
8. Schmidt MA, Michels VV, Dedwald GW. Cases of neurofibromatosis with rearrangements of chromosome 17 involving band 17q11.2. Am J Hum Genet 1987; 28: 771-7. [ Links ]
9. Thomas G, Mérel P, Sanson M, Hoang-Xuan K, Zucman J, Desmaze C, et al. Neurofibromatosis type 2. Eur J Cancer 1994; 30: 1981-7. [ Links ]
10. Eyden B, Chorneyko KA, Shanks JH, Menasce LP, Barnerjee SS. Contribution of electron microscopy to understanding cellular differentiation in mesenchymal tumours of the gastrointestinal tract. Ultrastruct Pathol 2002; 26: 269-85. [ Links ]
11. Walker P, Dvorak AM. Gastrointestinal autonomic nerve (GAN) tumour: Ultrastructural evidence for a newly recognized entity. Arch Pathol Lab Med 1986; 110: 309-16. [ Links ]
12. Herrera GA. Small bowel neoplasm. Dig Dis Sci 1985; 30: 698. [ Links ]
13. Hohenberger P, Wardelmann E. Gastrointestinale stromatumoren. Chirurg 2006; 77: 33-44. [ Links ]
14. Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology 1991; 19: 1-11. [ Links ]
15. Koeppen S, Wejda B, Dormann A, Hoffmeister D, Stolte M, Huchzermeyer H. Gastrointestinale stromatumoren (GIST) des jejunums bei einer Patientin mit neurofibromatose typ 1 (Morbus von Recklinghausen). Z Gastroenterol 2004; 42: 1183-7. [ Links ]
16. Kramer K, Siech M, Sträter J, Aschoff AJ, Henne-Bruns D. Hämorrhagischer Schock bei Doppelmalignom: Gastrointestinaler Stromatumor (GIST) und neuroendokrines Karzinom bei Morbus Recklinghausen. Z Gastroenterol 2005; 43: 281-8. [ Links ]
![]() Correspondence:
Correspondence:
Pablo Palma.
Sección de Coloproctología.
Servicio de Cirugía General y Aparato Digestivo.
Hospital Universitario Virgen de las Nieves.
18012 Granada.
e-mail: pablopalma@andaluciajunta.es
Recibido: 19-04-07.
Aceptado: 11-05-07