Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 no.2 Madrid Fev. 2009
The European contribution to "Sugarbaker's protocol" for the treatment of colorectal peritoneal carcinomatosis
Aportación europea al "protocolo de Sugarbaker" en el tratamiento de la carcinomatosis peritoneal colorrectal
A. Gómez Portilla, I. Cendoya, I. Olabarria, C. Martínez de Lecea, C. Gómez Martínez de Lecea, A. Gil, E. Martín, J. Muriel, L. Magrach, E. Romero, A. Lirola, N. Guede, N. Moraza, E. Fernández, M. Kvadatze, M. Valdovinos1, I. Larrabide1, N. Ruiz de Alegría2, J. L. Fernández2, C. Castillo2, O. Rua2 and M. A. Ulibarrena2
Departments of Surgery, 1Anesthetics, and 2Unit of Intensive Care. Peritoneal Carcinomatosis Programme. Hospital San José. Vitoria, Spain
ABSTRACT
Introduction: in 1981, Dr. PH Sugarbaker, challenging oncological orthodoxy, considered carcinomatosis to be a locoregional stage of the disease that was still susceptible to treatment with curative intent.
To this end he developed a new therapeutic alternative based on the combined treatment.
The macroscopic disease treated by maximum radical oncological cytoreductive surgery (through the peritonectomies described by him), followed by treatment of the residual microscopic disease with the direct intra-abdominal application of intraoperative chemotherapy with locoregional intensification, modulated by hyperthermia and early normothermic postoperative intra-abdominal chemotherapy.
Using this new therapeutic regimen, known as "Sugarbaker's Protocol", his group has reported 45% survival rates in carcinomatosis of colorectal origin at 5 years, and, in selected groups of patients, 50% survival rates at 5 years. The scientific community, however, has criticized these results considering that: it is a personal experience, with a not homogenous treatment protocol with developmental modifications over time, that it is a retrospective non-randomized study, and finally that the cytostatics used in his protocol are obsolete. Various European groups have replied to these main criticisms confirming the good results that this new therapeutic alternative offers for patients with carcinomatosis of colorectal origin. The purpose of this article is to present these contributions.
Material and methods: all the articles published in the English language by European groups in the world's medical literature have been reviewed using the Pubmed-MEDLINE database to identify the relevant articles related to the treatment of carcinomatosis of colorectal origin using cytoreduction and intraperitoneal chemotherapy from January 1980 to January 2008.
Results: the European contribution during these 25 years in favour of the "Sugarbaker's Protocol" has consisted fundamentally in: a) one multicenter retrospective study; b) two randomized prospective phase III studies; and c) the use of oxaliplatin and irinotecan as new cytostatic agents in the protocols for intraperitoneal chemotherapy.
At the same time, two new transcendental European contributions have been made in which the possibility has been considered of combined simultaneous treatment for patients with hepatic metastases and carcinomatosis, and the introduction, as a selection factor, of patients responsive to intravenous induction chemotherapy within the regimen of sandwich treatment (with systemic neoadjuvant and adjuvant chemotherapy) complementary to intraperitoneal chemotherapy.
Conclusions: the results obtained by European groups using "Sugarbaker's protocol" and "Elias' protocol" with oxaliplatin compel us to request that these treatments be considered by all professionals involved in the treatment of patients with colorectal carcinomatosis as the best treatment currently available for this condition. Furthermore a randomized, prospective, multicenter study should be carried out to clarify its value and the degree of scientific evidence. A validation of this treatment will change, in the future, the dogmatic consideration of carcinomatosis as an incurable disease stage.
Key words: Colorectal carcinomatosis. Cytoreductive surgery. Intraperitoneal chemotherapy. Hyperthermia.
RESUMEN
Introducción: el Dr. P. H. Sugarbaker en 1981, desafiando la ortodoxia oncológica, consideró la carcinomatosis como un estadio locorregional de la enfermedad susceptible todavía de tratamiento con intención curativa.
Para ello desarrolló una nueva alternativa terapéutica basada en el tratamiento combinado. La enfermedad macroscópica mediante la máxima cirugía citorreductora radical oncológica (merced a las peritonectomías por él descritas), seguido del tratamiento de la enfermedad microscópica residual con la aplicación directa intraabdominal, de quimioterapia de intensificación locorregional, intraoperatoria modulada por hipertermia y de quimioterapia intraabdominal normotérmica postoperatoria precoz. Con este nuevo esquema terapéutico, conocido como "Protocolo de Sugarbaker", su grupo ha publicado supervivencias en carcinomatosis de origen colorrectal de 45% a 5 años y en grupos selectos de pacientes supervivencia de 50% a 5 años. La comunidad científica, sin embargo, ha criticado estos resultados al considerar que: se trata de una experiencia personal, con un protocolo de tratamiento no homogéneo con modificaciones evolutivas en el tiempo, tratarse de un estudio retrospectivo no randomizado, y finalmente considerar que los citostáticos empleados en su protocolo son obsoletos. Diversos grupos europeos han dado respuesta a las principales objeciones, confirmando los buenos resultados que esta nueva alternativa terapéutica ofrece en pacientes con carcinomatosis de origen colorrectal. El objetivo de este trabajo es presentar estas aportaciones.
Material y métodos: se han revisado todos los artículos publicados en lengua inglesa por grupos europeos en la literatura médica mundial usando la base de datos Pubmed-MEDLINE para identificar los artículos relevantes relacionados con el tratamiento de la carcinomatosis de origen colorrectal mediante citorreducción y quimioterapia intraperitoneal desde enero de 1980 a enero de 2008.
Resultados: durante estos 25 años, la aportación europea como respuesta a las objeciones al "Protocolo de Sugarbaker" ha consistido fundamentalmente en: a) un estudio multicéntrico retrospectivo; b) dos estudios randomizados prospectivos fase III; y c) en la utilización del oxaliplatino e irinotecán como nuevos agentes citostáticos en los protocolos de quimioterapia intraperitoneal. Paralelamente se han producido dos nuevas aportaciones euro-peas trascendentales al considerar la posibilidad del tratamiento conjunto simultáneo en pacientes con metástasis hepáticas y carcinomatosis, y al introducir como factor de selección a los pacientes respondedores a quimioterapia intravenosa de inducción, dentro del esquema del tratamiento sándwich (con quimioterapia sistémica neoadyuvante y adyuvante) complementaria a la quimioterapia intraperitoneal.
Conclusiones: la resultados obtenidos por los grupos euro-peos utilizando el "protocolo de Sugarbaker" y el "protocolo de Elias" con oxaliplatino, nos obligan a solicitar que estos tratamientos sean considerados por todos los profesionales, involucrados en el tratamiento de pacientes con carcinomatosis colorrectal, como el mejor tratamiento disponible en la actualidad para esta patología, y permita la realización de un estudio randomizado prospectivo multicéntrico que esclarezca su valía y grado de evidencia científica. La validación de este tratamiento, permitirá en el futuro cambiar el dogma de considerar a la carcinomatosis como un estadio incurable de la enfermedad.
Palabras clave: Carcinomatosis colorrectal. Cirugía citorreductora. Quimioterapia intraperitoneal. Hipertermia.
Introduction
Dr. P. H. Sugarbaker, challenging oncological orthodoxy in 1981, considered carcinomatosis to be a locoregional disease stage still susceptible to treatment with curative intent (1). To this end he developed a new therapeutic alternative based on the combined treatment. The macroscopic disease treated by maximum radical oncological cytoreductive surgery (CS) (by means of the peritonectomies described by him) (2-4). Followed by treating residual microscopic disease with a direct intraabdominal application of intraperitoneal chemotherapy (IC) with locoregional intraoperative intensification, using hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) and/or administered under normothermia by means of early intraabdominal postoperative chemotherapy (5) (EPIC). With this new therapeutic regimen, known as "Sugarbaker's protocol", his group has published therapy results in patients with carcinomatosis of colorectal origin with survival rates of 45% at 5 years (6), and, in selected groups, of 50% at 5 years (7). The morbidity and mortality associated with this combined treatment is high. Although there is great variation in reported morbidity and mortality rates, with indices of 0-43% for the former and 0-20% for the latter (8-13), those of Sugarbaker's group still remain the best results with a morbidity of 25% and a mortality of 1.5% in carcinomatosis of colorectal origin. More recently other groups have improved these results in carcinomatoses of other origins (14). A majority of patients will suffer from abdominal or systemic recurrence, and will finally die from disease progression, but some may be recovered after a second or further cytoreduction (15,16). Some fortunate patients may exceptionally remain free of disease and be completely cured. The scientific community has criticized these results on the grounds of this being a personal experience with changes over time in the treatment protocol. Also, it is not based on a randomized prospective study, and finally some consider that the cytostatics used in his protocol are obsolete. However various European groups have answered these objections confirming the good results that this new therapeutic alternative offers to patients with carcinomatosis of colorectal origin. The purpose of this article is to present these contributions.
Material and methods
We reviewed all the articles published in the world's medical literature in English by European groups using the Pubmed-MEDLINE database to identify the relevant articles related to the treatment of carcinomatosis of colorectal origin using cytoreduction and intraperitoneal chemotherapy from January 1980 to January 2008; key words included: colorectal carcinomatosis, cytoreductive surgery, intraperitoneal chemotherapy, and hyperthermia.
Results
During these 25 years, the European contribution against objections to "Sugarbaker's Protocol" has consisted fundamentally in a multicenter retrospective study of the application of CS + IC, two randomized prospective phase III studies, and the use of oxaliplatin and irinotecan as new cytostatic agents in the protocols for IC.
At the same time, two new transcendental European contributions have come about, in which the possibility has been considered of combined simultaneous treatment for patients with hepatic metastases and carcinomatosis, and the introduction, as a factor for the selection, of patients responsive to intravenous induction chemotherapy within the regimen of sandwich treatment (with systemic neoadjuvant and adjuvant chemotherapy) complementary to IC.
Multicenter international study of the application of CS for the treatment of colorectal carcinomatosis
In 2004 Glehen et al. (17) published the results of a multi-institutional, international, retrospective study of the experience worldwide with this new alternative treatment in patients suffering from colorectal carcinomatosis treated with IC, excluding those with carcinomatosis originating in the appendix. Five hundred and six patients treated in 28 institutions with CS and perioperative IC made up the group for study. With an average follow-up of 53 months, the average survival rate was 19.2 months, with a 72% survival rate at one year, of 39% at three years, and of 19% at five years. Thirty-eight patients had survived for 5 years after cytoreduction, in spite of having diffuse peritoneal carcinomatosis. Cytoreduction extent was the chief determinant factor for the prognosis, as shown in table I.
Randomized, prospective phase-III studies of the application of intraperitoneal chemotherapy in the treatment of colorectal carcinomatosis
The main objection raised to "Sugarbaker's protocol" has been that studies were always retrospective and non-randomized. Only two randomized, prospective studies have been carried out and published so far on this subject (18,19). However, in spite of being ethically and scientifically correct, in the study carried out by the Institut Gustave Roussy, Paris (18), patients refused to be included in the random group for conventional treatment, which led to terminate the study when only 35 patients had been recruited. In spite of that, this study demonstrated the superiority of intraperitoneal chemotherapy over any other available treatment, with survival rates of 60% at 2 years, as well as the superiority of HIIC over EPIC. In the Netherlands Cancer Institute, a phase-III study was completed in patients with carcinomatosis of colorectal origin, which compared intraperitoneal chemotherapy versus systemic chemotherapy. In this study (19), 105 patients were included; the control group received conventional treatment with systemic 5 FU /leucovorin; and the study group received CS, and intraoperative intraperitoneal treatment was administered with mitomycin C (MMC) modulated by hyperthermia and followed by systemic chemotherapy with 5-FU/ leucovorin. In the study group, the grade of oncological cytoreduction achieved was complete (R1), without residual macroscopic disease, in only 18 patients (38%). Residual disease was less than 2.5 mm (R2a) in 21 patients (43%), and in 9 patients (19%) an adequate cytoreduction was not achieved (more than 2.5 mm of macroscopic disease left). Average survival was more than a year in both groups. The average survival was almost twice in the group with cytoreduction plus intraperitoneal chemotherapy as compared with the group treated with systemic chemotherapy (22.4 months compared to 12.6 months). These partial results, in spite of having been achieved with very poor cytoreductions (only 38% of the cytoreductions were the equivalent of complete cytoreduction, CC0-CC1) and having received intraperitoneal chemotherapy exclusively intraoperatively for 60 minutes with MMC, showed such important benefits that it was mandatory to terminate the study, as it would not have been ethically correct to offer treatments other than this new intraperitoneal therapeutic alternative. The grade of cytoreduction obtained was the main prognostic factor in this study. The long-term follow-up of these patients (20) showed survival rates of 75% at one year, 28% at three years, and 19% at 5 years, respectively. Such percentages cannot be reached with any of the presently available treatments.
New cytostatic agents, oxaliplatin and irinotecan, in the CS protocols
The use of oxaliplatin and irinotecan as new cytostatic agents in CS protocols has been developed and introduced by Dr. Elias' group in the Gustave Roussy Hospital, Paris (21-29). In spite of being administered exclusively during HIIC for only 30 minutes, but modulated by hyperthermia at 43 ºC, the effectiveness of these drugs was verified. In addition, in the studies with oxaliplatin, cytoreduction grade was the principal prognostic factor. Thus, when complete cytoreduction was achieved, survival was 83% at one year, 74% at 2 years, and 65% at 3 years. The use of intraperitoneal irinotecan has been hampered by high hematological toxicity in up to 58% of patients, which still necessitates a cautious approach to the use of this cytostatic (25,26). The most recent results published with "Elias' Protocol" using oxaliplatin show that, in the best of situations, complete cytoreduction-CC0, survival reaches 54% of patients at 5 years (27,28). These results are consistent with those published by Sugarbaker. The main advantage of this treatment regimen, known as "Elias' Protocol", is that it enables similar results to be attained without EPIC, even reducing postoperative complications and peritoneal recurrences (18).
Combined simultaneous treatment of patients with hepatic metastases and carcinomatosis
The presence of hepatic metastases has until now put a limit to the treatment of patients with peritoneal dissemination as this is considered to be systemic disease spread via the portal circulation. However, Elias (30-33) has demonstrated that occasionally the simultaneous approach to peritoneal disease in the course of surgery for hepatic metastases proved to be a treatment that was tolerated and effective when a complete cytoreduction of the disease was achieved. Twelve of their initial patients were treated by hepatectomy together with complete cytoreduction of the accompanying peritoneal disease and early postoperative intraperitoneal chemotherapy. There was no mortality or systemic complications from chemotherapy in their series. After an average follow-up of 14.4 months, no recurrence of the peritoneal disease was detected in any patients, and seven of them are free of disease. His position as a hepatic surgeon has created a favorable situation for the combination of hepatectomy and cytoreductive surgery in the treatment of advanced disease in colorectal cancer, and this may currently become a logical and feasible treatment. Some promising survival results have been achieved, 41.5% at 3 years and 26.5% at 5 years in patients with an average of 3.4 hepatic metastases (range 1 to 15) and a peritoneal carcinomatosis index of 12.4 (range 2-25) (33). Other groups have achieved similar results, with survivals of 44% at 4 years in patients treated simultaneously for carcinomatosis and hepatic metastases (34). Thus, we can claim today that the present limit for the treatment of colorectal disease is when the disease is confined to the abdominal cavity (locoregional recurrence, peritoneal dissemination or hepatic metastases), and surgery ensures a radical curative oncological resection of the disease; however, it should always be accompanied by treatment of the residual microscopic disease by the simultaneous administration of perioperative intraperitoneal chemotherapy.
Selection of patients responsive to intravenous induction chemotherapy
The criteria for inclusion of candidate patients in this new treatment have classically been as follows: patients aged between 18 and 75 years; with a good general condition, an ECOG of 1 or 2; without limiting comorbidities, this being understood to mean correct cardiorespiratory, hepatic and renal functioning; with a definitive diagnosis of abdominal disease confirmed by histological studies; with no extra-abdominal disease; not having received radiotherapy or chemotherapy within 30 days before cytoreduction, and with a signed informed consent (35). The great demand for this type of treatment, together with institutional limitations for its application, made it advisable to administer intravenous chemotherapy up to the time of applying the combined treatment. Disease progression in this waiting interval is a sign of a poor prognosis. And as with the treatment of hepatic metastases, patients who react well to neoadjuvant induction chemotherapy with a total or partial response are those who achieve best results. Complete cytoreduction is achieved in 90% of them, compared to only 52% in the group of non-responsive patients (36). This could be established as a new criterion in addition to classic criteria for the selection of candidates to receive this new therapeutic alternative, i.e., that patients undergoing neoadjuvant systemic treatment show no disease progression while they are waiting for cytoreductive surgery.
Discussion
There are few studies published on the natural history of carcinomatosis of colorectal origin. Average survival in patients suffering from colorectal carcinomatosis was 5.2 months in the multicenter EVOCAPE study by Sadeghi et al. (37), 6 months according to Chu et al. (38), and 9 months in the most important and recent series by Jayne et al. (39). Since the late fifties 5-FU has been practically the only effective chemotherapeutic option for patients with advanced colorectal cancer (40), and is still considered a basic drug. Although it only induces complete responses in 10% of patients, with an average survival between 9 and 12 months, and only 5% of the patients are alive 3 years later (41).
Five new drugs have changed the treatment horizon for advanced colorectal disease in the last decade: irinotecan, oxaliplatin, capecitabine, bevacizumab, and cetuximab. The combinations of 5-FU/LV with oxaliplatin or irinotecan (CPT-11) added to the target drugs are currently the most active treatments available in advanced colorectal cancer. However, average survival is up to 24 months (42-50). In spite of the advances in chemotherapeutic drugs, satisfactory treatments for peritoneal carcinomatosis are nowhere to be found. The main reason for these pessimistic results may be that cytostatic drugs do not reach peritoneal metastases in sufficiently high concentrations (51). In contrast to these results, in the worldwide multicenter study with "Sugarbaker's protocol", average survivals of 32.4 months were achieved when complete cytoreductions were carried out (17).
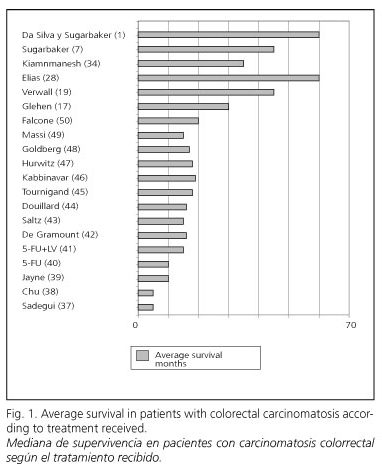
In the Phase-III study of the Netherlands Institute of Cancer, in the group with complete macroscopic cytoreduction and intraperitoneal chemohyperthermia the average survival was 42.9 months (19). Finally, Dr. Sugarbaker's group have reported an average survival of up to 60 months in a selected group of patients in which complete control of the macroscopic disease was obtained, as 50% of their patients have survived more than 5 years (1,7), as shown in figure 1.
There is no doubt that without treatment no patient suffering from carcinomatosis of colorectal origin can be expected to be alive 5 years after diagnosis. The same can be affirmed on patients treated with conventional regimens by systemic chemotherapy protocols; regardless of drugs, regimens, and doses employed, patients living longer than 5 years are anecdotic. However, both with "Elias' protocol" and with "Sugarbaker's protocol" these authors (7,27,28,52-54) have achieved survivals at 5 years of 54 and 50% respectively in selected groups of patients (Fig. 2), results that are unimaginable and unattainable with orthodox conventional treatments.
Conclusions
The results obtained by European groups using Sugarbaker's protocol and Elias' protocol with oxaliplatin compel us to request that these treatments be considered by all professionals involved in the treatment of patients with colorectal carcinomatosis as the best treatment available at present for this condition. Furthermore, a randomized, prospective, multicenter study should be carried out to clarify its worth and the degree of scientific evidence. The validation of this treatment will allow, in the future, to change the dogma of considering carcinomatosis as an incurable stage of disease.
References
1. Gómez da Silva R, Cabanas J, Sugarbaker PH. Limited survival in the treatment of carcinomatosis from rectal cancer. Dis Colon Rectum 2005; 48: 2258-63. [ Links ]
2. Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995; 221: 29: 42-7. [ Links ]
3. Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am 2003; 12: 703-27. [ Links ]
4. Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res 2007; 134: 247-64. [ Links ]
5. Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998; 14: 254-61. [ Links ]
6. Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis comparison of concomitant vs. delayed management. Dis Colon Rectum 2000; 43: 1341-8. [ Links ]
7. Sugarbaker PH. Peritoneal surface oncology: review of a personal experience with colorectal and appendiceal malignancy. Tech Coloproctol 2005; 9: 95-103. [ Links ]
8. Esquivel J, Vidal-Jove J, Steves MA, Sugarbaker PH. Morbidity and mortatily of cytoreductive surgery and intraperitoneal chemotherapy. Surgery 1993; 113: 631-6. [ Links ]
9. Jacquet P, Stephens AD, Averbach AM, Chang D, Ettinghausen SE, Dalton RR, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 1996; 77: 2622-9. [ Links ]
10. Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic heated intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999; 6: 790-6. [ Links ]
11. Shen P, Levine EA, Hall J, Case D, Russell G, Fleming R, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg 2003; 138: 26-33. [ Links ]
12. De Bree E, Witkamp A, Zoetmulder FAN. Intraperitoneal chemotherapy for colorectal cancer. J Surg Oncol 2002; 79: 46-61. [ Links ]
13. Loggie BW, Fleming RA. Complications of heated intraperitoneal chemotherapy and strategies for prevention. Cancer Treat Res 1996; 82: 221-33. [ Links ]
14. Kusumura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, et al. Cytoreductive surgery followed by intraoperative hyperthermic perfusion. Analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006; 106: 1144-53. [ Links ]
15. Gómez Portilla A, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal carcinomatosis from colorectal cancer: Analysis of prognostic features. World J Surg 1999; 23: 23-9. [ Links ]
16. Verwaal VJ, Boot H, Aleman BM, van Tinteren H, Zoetmulder FA. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: Location, treatment, and outcome. Ann Surg Oncol 2004; 11: 375-9. [ Links ]
17. Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004; 22: 3284-92. [ Links ]
18. Elias D, Delperro JR, Sideri L, Benhamou E, Pocard M, Baton O, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol 2004; 11: 518-21. [ Links ]
19. Verwaal VJ, Van Ruth S, De Bree E, Van Sloothen GW, Van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737-43. [ Links ]
20. Verwaal VJ, Van Ruth S, Witkamp A, Boot H, Van Sloothen GW, Zoetmulder FAN. Long term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005; 12: 65-71. [ Links ]
21. Elias D, Bonnay M, Puizillou JM, Antoun S, Dermirdjian S, El Otomany A, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Surg Oncol 2002; 13: 267-72. [ Links ]
22. Elias D, El Otomany A, Bonnay M, Paci A, Duvreux M, Antoun S, et al. Human pharmacokinetic study of heated intraperitoneal oxaliplatin in increasingly hypotonic solutions after complete resection of peritoneal carcinomatosis. Oncology 2002; 63: 346-52. [ Links ]
23. Elias D, Sideris L. Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg Oncol Clin N Am 2003; 12: 755-69. [ Links ]
24. Elias D, Pocard M, Sideris L, Ede C, Ducreux M, Boige V, et al. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal Carcinomatosis of colorectal origin. Br J Surg 2004; 91: 455-6. [ Links ]
25. Elias D, Matsuhisa T, Sideris L, Liberale G, Drouard-Troalen L, Raynard B, et al. Heated intra-operative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: Pharmacokinetics, tissue distribution and tolerance. Ann Oncol 2004; 15: 1558-65. [ Links ]
26. Elias D, Raynard B, Bonnay M, Pocard M. Heated intra-operative intraperitoneal oxaliplatin alone and in combination with intraperitoneal irinotecan: pharmacologic studies. EJSO 2006; 32: 607-13. [ Links ]
27. Elias D, Raynard B, Farkhondeh F, Goéré D, Rouquie D, Ciuchendea R, et al. Peritoneal carcinomatosis of colorectal origin. Long-term results of intraperitoneal chemohyperthermia with oxaliplatin following complete cytoreductive surgery. Gastroenterol Clin Biol 2006; 30: 1200-4. [ Links ]
28. Elias D, Benizri E, DiPietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol 2007; 14: 509-14. [ Links ]
29. Elias D, Pocard M, Goere D. HIPEC with oxaliplatin in the treatment of peritoneal carcinomatosis of colorectal origin. Cancer Treat & Research 2007; 134: 303-18. [ Links ]
30. Elias D, Dube P, Bonvalot S, Meshaka P, Manai M, Cavalcanti A et al. Treatment of liver metastases with moderate peritoneal carcinomatosis by hepatectomy and cytoreductive surgery followed by immediate post-operative intraperitoneal chemotherapy: feasibility and preliminary results. Hepato-Gastroenetrol 1999; 46: 360-3. [ Links ]
31. Elias D, Goharin A, El Otamany A, Bonvalot S, Meshaka P, et al. Treatment of liver metastases associated with moderate peritoneal carcinomatosis by hepatectomy and cytoreductive surgery followed by immediate intraperitoneal chemotherapy: Results in 22 cases. J Surg Invest 2001; 3: 31- 6. [ Links ]
32. Elias D, Sideris L, Pocard M, Ouellet JF, Boige V, Lasser Ph, et al. Results in R0 resection for colorectal liver metastases associated with extrahepatic disease. Ann Surg Oncol 2004; 11: 274-80. [ Links ]
33. Elias D, Benizri E, Pocard M, Ducreux M, Boige V, Lasser P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. EJSO 2006; 32: 632-6. [ Links ]
34. Kiammanesh R, Scaringi S, Sabater JM, Caste B, Pons-Kerjean N, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg 2007; 245: 597-605. [ Links ]
35. Gómez Portilla A. Carcinomatosis peritoneal. Diez años aplicando la nueva triple terapia combinada. Experiencia personal. Cir Esp 2007; 82: 346-51. [ Links ]
36. Elías D, Benizri E, Vernerey D, Eldweny H, Dipietrantonio D, Pocard M. Preoperative criteria of incomplete resectability of peritoneal carcinomatosis from non-appendiceal colorectal carcinoma. Gastroenterol Clin Biol 2005; 29: 1010-3. [ Links ]
37. Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivpoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecological malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000; 88: 358-63. [ Links ]
38. Chu DZJ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecological malignancy: a prospective study of prognostic factors. Cancer 1989; 63: 364-7. [ Links ]
39. Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002; 89: 1545-50. [ Links ]
40. Massacesi C, Pistilli B, Valeri M, Lippe P. Rocchi MBL, Cellerino R, et al. Predictors of short-term survival and progression to chemotherapy in patients with advanced colorectal cancer treated with 5-fluorouracil based regimens. Am J Clin Oncol 2002; 25: 140-8. [ Links ]
41. Anonymous: Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced colorectal cancer meta-analysis project. J Clin Oncol 1992; 10: 896-903. [ Links ]
42. De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938-47. [ Links ]
43. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343: 905-14. [ Links ]
44. Douillard JY, Cunningham D, Roth AD, Navarro M, Karasek P, Jandik P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer. A multicenter randomised trial. Lancet 2000; 355: 1041-7. [ Links ]
45. Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229-37. [ Links ]
46. Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005; 23: 3697-705. [ Links ]
47. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335-42. [ Links ]
48. Goldberg RM, Sargent DJ, Morton RE, Fuchs ChS, Ramanatham RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22: 23-30. [ Links ]
49. Masi G, Marcucci L, Loupakis F, Cerri E, Barbara C, Bursi S, et al. First-line 5-fluorouracil/folinic acid, oxaliplatin and irinotecan (FOLFOXIRI) does not impair the feasibility and the activity of second line treatments in metastatic colorectal cancer. Ann Oncol 2006; 17: 1249-54. [ Links ]
50. Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007; 25: 1670-6. [ Links ]
51. Yonemura Y, Fujimura T, Fushida S, Takegawa S, Kamata T, Katayama K, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg 1991; 15: 530-6. [ Links ]
52. Sugarbaker PH. Colorectal carcinomatosis: a new oncologic frontier. Current Opinion in Oncology 2005; 17: 397-9. [ Links ]
53. Sugarbaker PH. A curative approach to peritoneal carcinomatosis from colorectal cancer. Seminars in Oncology 2005; 32: S68-73. [ Links ]
54. Da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 2006; 203 (6): 878-86. [ Links ]
![]() Correspondence:
Correspondence:
A. Gómez Portilla.
Director of the Peritoneal Carcinomatosis Programme.
Hospital San José.
C/ Beato Tomás de Zumárraga, 10.
01008 Vitoria, Spain.
e-mail: agomezpor@teleline.es
Received: 12-09-08.
Accepted: 27-01-09.











 texto em
texto em