Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 no.5 Madrid Mai. 2009
Prospective evaluation of liver fibrosis in chronic viral hepatitis C infection using the Sabadell NIHCED (non-invasive hepatitis C related cirrhosis early detection) index
Evaluación de la fibrosis hepática en la hepatitis crónica por virus C mediante la aplicación prospectiva del Sabadell´s NIHCED score (Sabadell´s Non Invasive, Hepatitis C Related-Cirrhosis Early Detection Score)
G. Bejarano1,2, M. Vergara1,2, M. Gil1,2, B. Dalmau1,2, J. Puig2,3, M. R. Bella4, D. Suárez5 and X. Calvet1,2
1Service of Digestive Diseases. 2CIBERehd. Instituto Carlos III.3Unit of High Technology Diagnosis. 4Service of Anatomy.5Unit of Stadistics and Epidemiology. Universitat Autònoma de Barcelona. Corporació Sanitària Parc Taulí. Sabadell, Barcelona. Spain
Financial support: this study was funded, in part, by a CIR grant form Corporaciò Parc Taulí, Código CEIC #2004112 and by Instituto de Salud Carlos III (C03/02 and PI 05/1157 and CIBERehd).
ABSTRACT
Introduction: liver disease resulting from chronic hepatitis C virus (HCV) infection follows an asymptomatic course towards cirrhosis and its complications in 20-40% of cases. Earlier studies demonstrated that advanced fibrosis is a prognostic factor. The "gold standard" for the evaluation of fibrosis grade is liver biopsy. Our group validated a predictive index - NIHCED - based on demographic, laboratory parameters, and echoghraphic data to determine the presence of cirrhosis.
Objective: our objective is to evaluate whether the NIHCED score predicts the presence of advanced fibrosis in patients with chronic HCV infection.
Material and methods: this prospective study included patients with chronic HCV infection who underwent liver biopsy and were administered the NIHCED score. Fibrosis grade correlated with the NIHCED score using the ROC curve analysis and Spearman's correlation coefficient.
Results: in total 321 patients were included (male/female ratio 1.27) with a mean age of 48 ± 14 years. Liver biopsy showed that 131 (30.5%) had no fibrosis or had portal expansion while 190 (69.5%) had advanced fibrosis or cirrhosis. At a cut-off point of 6, sensitivity was 72%, specificity was 76.3%, positive predictive value (PPV) was 81%, negative predictive value (NPV) was 63.7%, and diagnostic accuracy was 72.5%, with an area under the curve (AUC) of 0.787, and a Spearman's correlation coefficient of r = 0.65.
Conclusions: the NIHCED score predicts the presence of advanced fibrosis in an elevated percentage of patients with a need of liver biopsy.
Key words: Hepatitis C virus liver disease. Advanced fibrosis. Predictive index. HIHCED index.
RESUMEN
Introducción: la hepatitis crónica por VHC cursa de forma asintomática desarrollando cirrosis hepática y sus complicaciones en un 20-40% de los casos. En estudios previos se ha demostrado que la fibrosis avanzada es un factor pronóstico fundamental. El método gold standard para la valoración del grado de fibrosis es la biopsia hepática. Nuestro grupo ha validado un índice predictivo, el NIHCED (Sabadell's Non Invasive, Hepatitis C related-Cirrosis Early Detection Score), basado en datos demográficos, analíticos y ecográficos para determinar la presencia de cirrosis.
Objetivo: nuestro objetivo es el de evaluar si el NIHCED predice la presencia de fibrosis avanzada en los pacientes con hepatitis crónica por virus C.
Material y métodos: estudio prospectivo donde se incluyeron pacientes con hepatitis crónica por VHC. Se les realizó una biopsia hepática y el NIHCED. El grado de fibrosis se correlacionó con el valor del NIHCED mediante curva de ROC y el coeficiente de correlación de Spearman.
Resultados: se incluyeron un total de 321 pacientes (ratio hombre/mujer 1,27) con una edad media de 48 ± 14 años. La biopsia hepática mostró que 131 (30,5%) no tenían fibrosis o era expansión portal, mientras que 190 (69,5%) tenían fibrosis avanzada o cirrosis. Para un punto de corte de 6 puntos, la sensibilidad fue del 72%, especificidad del 76,3%, VPP del 81%, VPN del 63,7% y una precisión diagnóstica del 72,5%, con un área bajo la curva fue de 0,787 y un coeficiente de correlación de Spearman de r = 0,65.
Conclusiones: el NIHCED predice la presencia de fibrosis avanzada en un elevado porcentaje de pacientes sin necesidad de realizar biopsia hepática.
Palabras clave: Hepatitis por virus C. Fibrosis avanzada. Índice predictivo. Índice NIHCED.
Abbreviation list
HVB: Hepatitis B virus.
HIV: Human immunodeficiency virus.
TSI: Transferrin saturation index.
ANAs: Anti-nuclear antibodies.
PBC: Primary biliary cirrhosis.
AP: Alkaline phosphatase.
AMAs: Anti-mitochondrial antibodies.
GGT: Gamma-glutamyl transpeptidase.
ALT: Alanine transaminase.
AST: Aspartate aminotransferase.
Introduction
Currently, viral hepatitis C (HCV infection) constitutes the most prevalent cause of chronic hepatitis in developed countries. It is estimated that 3% of the world's population (170 million people) suffer from chronic HCV infection. In Spain, the prevalence has been calculated between 1.6 and 2.6% of the population, and increases progressively with age to reach 4.1% in those > 65 years (1,2). Chronic disease evolves asymptomatically and, as such, is not detected in many cases.
Approximately 80% of those infected with HCV are chronic virus carriers (3,4), one third of them develop a liver crisis in the subsequent 20 years, and up to 7% of patients with cirrhosis develop hepatocellular carcinoma (4,5). However, approximately two thirds of chronic carriers present with a clinically asymptomatic infection that, in the absence of other adjuvant factors (alcohol consumption, HVB co-infection and/or HIV), will remain stable or progress slowly to hepatic fibrosis (6).
Several studies have shown that host factors, rather than viral factors, have a greater impact on the progression of hepatic lesions. Specifically, age, alcohol consumption and male gender appear to have a greater prognostic value versus genotype or viral load (7).
The "gold standard" diagnostic method to evaluate chronic HCV infection grade is liver biopsy. Some authors recommend periodic liver biopsies (8) in patients with chronic HCV infection. However, serial liver biopsies are difficult because of their associated morbidity (3%), mortality (0.03%), and low patient acceptance (9). However, it is important to detect early liver cirrhosis in order to refer these patients for appropriate follow-up, and to detect complications.
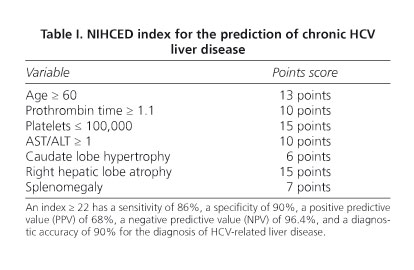
To date, several predictive markers of liver cirrhosis that include clinical, laboratory, and echographic data have been published (10-19). In our Unit we developed and validated an index predictive of chronic HCV-related hepatitis. Termed the Sabadell NIHCED (Non-Invasive, Hepatitis C-related Cirrhosis Early Detection) index, it includes several demographic, laboratory and echographic parameters (20) (Table I). A score ≥ 22 has a sensitivity of 86%, a specificity of 90%, a positive predictive value (PPV) of 68%, a negative predictive value (NPV) of 96.4%, and a diagnostic accuracy of 90% (20).
The goal of the present study was to evaluate the sensitivity, specificity, PPV, NPV, and diagnostic precision of NIHCED to determine the presence of advanced fibrosis in patients with chronic liver disease due to HCV.
Material and methods
The study is single-centered and prospective with patients diagnosed as having hepatitis due to HCV. The study was conducted at Hospital Parc Taulí, Sabadell (Barcelona), this being a District General Hospital serving a catchment area of 11 municipalities with responsibility for healthcare provision for 414,152 citizens.
Patient selection
Between January 1993 and July 1999 patients were prospectively and consecutively recruited from among those with HCV-associated liver disease being evaluated in the Liver Disease Unit of "Hospital Parc Taulí" (Sabadell, Barcelona).
Inclusion criteria
-Asymptomatic patients diagnosed as having hepatitis due to HCV with elevated blood levels of HCV-RNA.
-Patients who consented to have a liver biopsy to establish the grade of fibrosis.
Exclusion criteria
-Presence of any disease that has a concomitant liver involvement: hemochromatosis, Wilson's disease, chronic hepatitis due to HVB, drug-induced hepatotoxicity, HIV positivity, auto-immune hepatitis, alcoholic hepatitis. All patients had blood tests for VHB and HIV, hemochromatosis assessment (iron, ferritin, TSI), auto-immune hepatitis (ANAs, anti-smooth muscle cell antibodies), PBC (AP, AMAs), Wilson's disease (ceruloplasmin), and α-1 antitrypsin (antitrypsin levels).
-Presence of other disease with poor short-term prognosis (disseminated neoplasia, advanced renal insufficiency...).
-Cirrhosis already established (presence of portal hypertension in the form of esophageal varices, ascites or hepatocarcinoma).
-Consumption of alcohol > 20 g/d, or other drugs.
The study protocol was approved by the Ethics Committee of Hospital "Parc Taulí", Sabadell (Barcelona), and designed according to the ethical guidelines of the 1975 Declaration of Helsinki (revised in 1983).
Clinical assessment
A total of 321 patients were included. A clinical history and detailed physical examination were performed. Once accepted into the study a blood sample was taken for laboratory analysis, and a biopsy was performed to determine the fibrosis grade resulting from HCV infection.
Laboratory testing
The following measurements were performed: complete hemogram with total leukocyte count including percentage neutrophils and lymphocytes, hemoglobin, mean corpuscular volume, platelet count, prothrombin time, albumin (mg/dL), total and direct bilirubin (U/L), creatinine (mg/dL), urea (mg/dL), AP (U/L), GGT (U/L), ALT (U/L), AST (U/L), AST/ALT ratio, and gamma globulins.
Hepatic echography
A Doppler-echography of the liver analyzed 13 echographic variables. The variables included in the NIHCED index and statistically related to liver fibrosis grade were:
-Hypertrophy of the caudate lobe (diameter > 4 cm).
-Right hepatic lobe atrophy (longitudinal diameter < 9 cm).
-Splenomegaly (> 13 cm).
Echograms were performed with a Toshiba 140 and Aloka SSD-650 equipment (Toshiba Medical Systems, Zoetermer, Netherlands; Aloka España SL, Madrid; Spain), with 3.5 or 3.75 MHz. All tests were performed by a single experienced echographer at the UDIAT Center of Hospital Parc Taulí, Sabadell.
The NIHCED score was calculated for each patient using data obtained from laboratory tests and hepatic echograms. The maximum time lapse between NIHCED index determination and liver biopsy was 3 months.
Liver biopsy
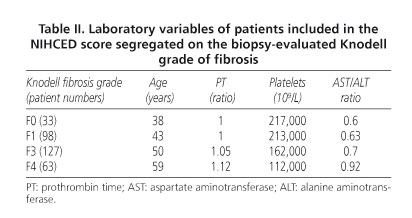
All patients had an echographically-guided liver biopsy performed using a tru-cut needle. The biopsy sample was fixed in FAA (formaldehyde, alcohol, acetic acid) and embedded in paraffin. The mean size of biopsy samples measured 11.6 mm, with a mean number of fibrous tracts of 12.2. Sample tissue was stained with hematoxylin-eosin, Masson trichrome stain, and 1% picrosirius red solution. All samples were evaluated by an experienced pathologist at the Pathology Department, Hospital "Parc Taulí", Sabadell. The pathologist was blinded with respect to the provenance of samples and laboratory results of patients. Fibrosis grade was scored on the Knodell scale, which classifies fibrosis into four stages (F0, absence of fibrosis; F1, fibrosis expansion; F3, porto-portal fibrosis bridges; F4, cirrhosis). Stages ≥ F3 are considered "advanced fibrosis".
Statistical analyses
Data obtained were processed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, USA).
Student's t-test was used for comparison between continuous variables. When distribution normality could not be assumed, or when sample size was < 10 observations in any group, the Mann-Whitney U test was applied. Comparison of proportions was performed with the χ2 test for categorical variables. When the 2 x 2 contingency table of expected values was < 5, Fisher's exact test was applied. Correlations of quantitative variables (NIHCED score) with ordinal variables (liver fibrosis, Knodell index) were tested using Spearman's ρ coefficient. A value of p < 0.05 was considered statistically significant.
The Knodell grade of fibrosis was correlated with the NIHCED score using the receiver operating characteristic - ROC - curve analysis, from which sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to differentiate patients into two groups: those with an absence of histologically demonstrable fibrosis (F0) or only one fibrous portal expansion (F1), and those who presented with advanced fibrosis (F3) or established cirrhosis (F4) according to the Knodell index.
Results
Demographic data
A total of 321 patients were included. Mean age was 48 ± 14 years, with a male-to-female ratio of 1.27.
Analysis of the NIHCED score as a predictor of advanced fibrosis in patients with chronic HCV infection
All 321 patients were segregated into two groups: those with an index of non-significant fibrosis (Knodell F0 and F1), for a total of 131 patients (30.5%), and those with advanced fibrosis (Knodell F3 and F4), for a total of 190 patients (69.5% of the total).
Table II summarizes the laboratory values included in the NIHCED index according to fibrosis grade in the biopsy sample. Table III summarizes the echographic data of the NIHCED score according to fibrosis grade.
From the ROC curve analysis a score > 6 was selected as providing the best combination of sensitivity, specificity and diagnostic accuracy in detecting advanced fibrosis (Knodell F3 and F4). Sensitivity was 72%, specificity 75%, PPV 81%, NPV 63.7%, and diagnostic accuracy 72.5%. The area under the curve (AUC) was 0.79 (95% CI: 0.74-0.84). The positive and negative predictive values of the likelihood ratio indicated an LR (+) of 3.04 and LR (-) of 0.37 (Fig. 1).
The correlation between the Knodell fibrosis index and the NIHCED score had a Spearman's correlation coefficient of r = 0.65.
NIHCED score as predictive of advanced fibrosis: estimation cohort and validation cohort
All 321 patients were divided randomly into two groups, the estimation cohort and the validation cohort, composed of 217 and 104 patients, respectively.
ROC curve analyses were applied to both groups with a cutoff score of > 6. The estimation cohort had a sensitivity of 70%, specificity of 74%, and diagnostic accuracy of 72% with an AUC of 0.77 (95% CI: 0.71-0.83). The calculation of the positive and negative predictive values of the likelihood ratio showed an LR (+) of 2.7 and LR (-) of 0.40.
The validation cohort showed a sensitivity of 76%, specificity of 76.3%, and diagnostic accuracy of 76% with an AUC of 0.82 (95% CI: 0.74-0.90). The calculation of the positive and negative likelihood ratio showed an LR (+) of 3.21 and LR (-) of 0.31 (Table IV).
Discussion
Several authors have developed non-invasive indices for the diagnosis of cirrhosis or of advanced fibrosis in order to avoid the need for liver biopsy (21-23). Liver biopsy continues to be the "gold standard" for fibrosis assessment despite the method having a considerable percentage of false positive and false negative results from sample errors and inter-observer differences.
Despite all these shortcomings, and the availability of several predictive indices, there has been no substitute for biopsy as ideal method as yet. Some authors have suggested combining several indices as a non-invasive method of diagnosis (24,25) even though there are some cases that continue to need liver biopsy for complete characterization. Leroy et al. (24) compared the histology results obtained in 180 chronic HCV infection patients with 6 different non-invasive indices. Their results showed that only one third of patients remained correctly categorized when combining the FibroTest (26) and the APRI (22). Castera et al. (27) highlighted that when FibroTest results were combined with that of transient elastometry findings coincided for 70-80% of patients, and liver biopsy could be avoided for 77% of patients with advanced fibrosis.
All non-invasive indices can detect extreme values with sufficient certainty, but only offer partial information on liver fibrosis for patients with intermediate values, and thus liver biopsy remains the "gold standard".
A considerable inconvenience of some of these non-invasive indices such as the FibroTest (28) is that they have not been done on a routine basis, given that they provide a mathematical formula that combines, for instance, 5 variables (FibroTest): total bilirubin, GGT, haptoglobin, alpha 2-microglobulin, and apolipoprotein A1. As such, this index is difficult to apply in the majority of routine clinical practices. Furthermore, the high economic cost of these tests means that they cannot be performed routinely in patients with chronic HCV infection.
A group at the University of Sydney (29) has proposed a non-invasive model of fibrosis progression that includes age, AST, cholesterol, alcohol use, and insulin resistance (30). Romera et al. validated the Sydney index in patients with hepatitis and showed a negative predictive value of 70%, as compared to the 93% communicated by the authors (31). As such, it would appear that adding the high-cost insulin resistance test to this index would provide a greater benefit to the NIHCED index as generated and validated by our group.
The NIHCED score, as with other non-invasive indices such as that of Forns (21), uses parameters that are routinely obtained in clinical practice to evaluate the follow-up of patients with chronic HCV infection. Chronic HCV infection, as has been commented upon earlier, is a highly prevalent infection affecting 2-3% of the Spanish population. This implies that exhaustive follow-up by specialists of all infected patients would nearly collapse hepatology specialist healthcare.
Due to the infection often being asymptomatic, many of these patients are not identified until their diagnosis in the course of other explorations. Conversely, a large group of asymptomatic patients are young with an active work life but are not monitored on a regular basis, or the monitoring is left to the family doctor or primary-care physician. The NIHCED score was designed with the intention of offering an attainable tool to the family doctor that is easy to score and to evaluate, and which serves to detect advanced fibrosis early. Applying the tool would identify a subgroup of patients with advanced fibrosis who would need to be referred for specialist monitoring. Many authors have demonstrated that advanced fibrosis is a fundamental diagnostic factor in patients with chronic HCV infection. Its presence in a biopsy would indicate that progression in these patients is faster than in those with no fibrosis or only fibrous expansion in their biopsy samples (32).
The NIHCED score contains the liver US data that add prognostic factors to the index. Of note, in 27% of patients newly diagnosed with hepatocarcinoma there is no previous evidence of advanced fibrosis or of cirrhosis and it is a liver echogram that guides the diagnosis on which to initiate treatment (33). One shortcoming that can occur in the development and validation of an index incorporating echographic data is that of an inter-observer bias. In the NIHCED score only one experienced radiologist performed all echograms and evaluated all sonographic findings.
The results of our study indicate that all patients > 65 years of age have a high probability of presenting with advanced fibrosis. Previous studies evaluating this conclusion identified that one of the main factors influencing disease progression was clinical evolution time, which is one that clearly increases with age (34-36).
The combination of laboratory and echographic data is of value because, in patients in whom laboratory parameters are within the normal range, the radiological data included in our index may be of greater value. Caudate lobe hypertrophy, splenomegaly, or right hepatic lobe atrophy would suggest, with a high degree of sensitivity and specificity, that a patient has advanced fibrosis.
Currently, there are gaps in the use of these non-invasive methods. One of the few studies in patients with hepatitis not caused by HCV (i.e., we know how these indices function in patients infected with HCV) indicated that other mechanisms exist that induce hepatic lesions such as hepatitis B or alcohol, but that reliability is unknown. The ability of the NIHCED score to distinguish between advanced fibrosis and absence of fibrosis or portal expansion (cutoff score > 6) has a sensitivity of 72%, specificity of 76.3%, PPV of 81%, NPV of 63.7%, and diagnostic accuracy of 72.5% with an AUC of 0.79 (95% CI: 0.74-0.84). These results are as good, if not better, as those obtained with other non-invasive methods.
In conclusion, the NIHCED score is as effective as other non-invasive indices for determining the presence of advanced fibrosis. The addition of echographic data introduces prognostic factors into the information obtained. In comparison to other non-invasive methods, the HIHCED score is simple and easy to apply in any outpatient clinic, whether specialist or primary care.
Future studies are needed to evaluate the utility of these non-invasive methods for the long-term follow-up of patients with chronic HCV infection.
![]() Correspondence:
Correspondence:
Guillermina Bejarano-Redondo.
Servicio de Digestivo. Hospital de Sabadell.
Institut Universitari Parc Taulí. Universitat Autònoma de Barcelona.
Parc Taulí, s/n. 08208 Sabadel. Barcelona, Spain.
e-mail: gbejarano@tauli.cat
Received: 04-11-08.
Accepted: 24-02-09.
References
1. Domínguez A, Bruguera M, Vidal J, Plans P, Salleras L. Community-based seroepi-demiological survey of HCV infection in Catalonia, Spain. J Med Virol 2001; 65: 688-93. [ Links ]
2. Riestra S, Fernández E, Leiva P, García S, Ocio G, Rodrigo L. Prevalence of hepatitis C virus infection in the general population of northern Spain. Eur J Gastroenterol Hepatol 2001; 13: 477-82. [ Links ]
3. Di Bisceglie AM. Hepatitis C. Lancet 1998; 9099: 351-5. [ Links ]
4. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 2000; 20(1): 17-35. [ Links ]
5. Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology 1997; 26(3 Supl. 1): 34S-38S. [ Links ]
6. Alberti A, Vario A, Ferrari A, Pistis R. Chronic hepatitis C - natural history and cofactors. Aliment Pharmacol Ther 2005; 22 (Supl. 2): 74-8. [ Links ]
7. Poynard T, Bedossa P, Opolon P, for the OBSVRIC, METAVIR, CLINIVIR and DOSVIRC group. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997; 349: 825-32. [ Links ]
8. Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology 2002; 36: S152-S160. [ Links ]
9. Garcia-Tsao G, Boyer JL. Outpatient liver biopsy: how safe is it? Ann Intern Med 1993; 118: 150-3. [ Links ]
10. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998; 93: 44-8. [ Links ]
11. Reedy DW, Loo AT, Levine RA. AST/ALT ratio > 1 is not diagnostic of cirrhosis in patients with chronic hepatitis C. Digestive Dis Sci 1998; 43: 2156-9. [ Links ]
12. Kaul V, Friedenberg FK, Braitman LE, Anis U, Zaeri N, Fazili J, et al. Development and validation of a model to diagnose cirrhosis in patients with hepatitis C. Am J Gastroenterol 2002; 97: 2623-8. [ Links ]
13. Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113: 1609-16. [ Links ]
14. Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002; 36: 986-92. [ Links ]
15. Bonacini M, Hadi G, Govindarajan S, Lindsay L. Utility of a discriminant índex for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1997: 92; 1302-4. [ Links ]
16. Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection- Analysis of 300 cases. Radiology 2003; 227: 89-94. [ Links ]
17. Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30: 472-8. [ Links ]
18. Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D´Errico A, et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol 1997; 27: 979-85. [ Links ]
19. Gomez, de la Cámara A. All that neurologists would like to know about sensitivity, specificity and predictive values. Neurología 2003; 18 (Supl. 2): 11-8. [ Links ]
20. Obrador BD, Prades MG, Gómez MV, Domingo JP, Cueto RB, Rué M, et al. A predictive index for the diagnosis of cirrhosis in hepatitis C based on clinical, laboratory, and ultrasound findings. Eur J Gastroenterol Hepatol 2006: 18: 57-62. [ Links ]
21. Forns X, Ampurdanes S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002; 36: 986-92. [ Links ]
22. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518-26. [ Links ]
23. Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology 2005; 42: 282-92. [ Links ]
24. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003; 39: 239-44. [ Links ]
25. Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46: 775-82. [ Links ]
26. Sebastiani G, Vario A, Guido M, Noventa F, Plebani M, Pistis R, et al. Stepwise combination algorithms of non-invasive markers to diagnose significant fibrosis in chronic hepatitis C. J Hepatol 2006; 44: 686-93. [ Links ]
27. Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005; 128: 343-50. [ Links ]
28. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357: 1069-75. [ Links ]
29. Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, et al. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology 2004; 39: 1239-47. [ Links ]
30. Fartoux L, Poujol-Robert A, Guechot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut 2005; 54: 1003-8. [ Links ]
31. Romera M, Corpas R, Romero-Gómez M. La resistencia a la insulina en la valoración no invasiva de la fibrosis en pacientes con hepatitis C: Estudio comparativo de métodos bioquímicos. Rev Esp Enferm Dig 2006; 98: 161-9. [ Links ]
32. Alberti A, Chemello L, Benvegnù. Natural history of hepatitis C. EASL Internacional Consensus Conference on Hepatitis C. J Hepatol 1999; 31(Supl. 1): 17-24. [ Links ]
33. Vergara M, Gil M, Dalmau B, Ribot R, Navarro C, Martín A, et al. Historia natural del carcinoma hepatocelular en una cohorte de pacientes de un hospital comarcal. Rev Esp Enferm Dig 2008; 100: 682-7. [ Links ]
34. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with non-alcoholic steatohepatitis. Hepatology 1999; 30: 1356-62. [ Links ]
35. Alberti A, Vario A, Ferrari A, Pistis R. Chronic hepatitis C - natural history and cofactors. Aliment Pharmacol Ther 2005; 22(Supl. 2): 74-8. [ Links ]
36. Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral and environmental factors. JAMA 2000; 284: 450-6. [ Links ]











 texto em
texto em