Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.102 no.2 Madrid Fev. 2010
Macroscopic small bowel mucosal injury caused by chronic nonsteroidal anti-inflammatory drugs (NSAIS) use as assessed by capsule endoscopy
Valoración mediante cápsulas endoscópicas de las lesiones intestinales mucosas causadas por antiinflamatorios no esteroideos (AINE)
A. Caunedo-Álvarez, B. J. Gómez-Rodríguez, J. Romero-Vázquez, F. Argüelles-Arias, R. Romero-Castro, J. M. García-Montes, F. J. Pellicer-Bautista and J. M. Herrerías-Gutiérrez
Service of Gastroenterology. University Hospital Virgen Macarena. Sevilla, Spain
ABSTRACT
Objective: to evaluate the type, frequency, and severity of macroscopic small bowel mucosal injury after chronic NSAID intake as assessed by capsule endoscopy (CE), as well as to correlate the severity of gastroduodenal and intestinal damage in these patients.
Material and methods:a prospective, endoscopist-blind, controlled trial. Sixteen patients (14F/2M; age: 57.06 ± 10.16 yrs) with osteoarthritis (OA) on chronic therapy with NSAIDs underwent CE and upper gastrointestinal endoscopy (UGE). Seventeen patients with OA (9F/2M; age: 57.47 ± 9.82 yrs) who did not take NSAIDs were included as a control group. A scale ranging from 0 to 2 (0 = no lesions, 1-minor = red spots or petechiae, denuded areas and/or 1-5 mucosal breaks; 2-major = > 5 mucosal breaks and/or strictures, or hemorrhage) was designed to assess the severity of small bowel mucosal injuries.
Results: CE found intestinal lesions in 75% (12/16) of patients in the study group and in 11.76% (2/17) of controls (p < 0.01). Seven out of 16 NSAID consumers (43.75%) and none in the control group (0%) had a major small bowel mucosal injury (p < 0.01). The percentages of patients with grade 1 and 2 gastroduodenopathy in the study group, as assessed by UGE, were 37.14 and 23.81%, respectively. There was no significant difference in the rate of major enteropathy between patients with none or minor gastroduodenal injury, and those with major gastroduodenopathy (43.75 vs. 40%; p = N.S.).
Conclusions: chronic NSAID intake is associated with a high rate of small bowel mucosal injuries. Our data have failed to demonstrate a relationship between the severity of gastroduodenal and intestinal injury.
Key words: NSAID. Small bowel mucosal injury. Capsule endoscopy. Enteropathy. Prospective. Controlled.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are associated with a significant risk of gastrointestinal events with clinical and economic consequences (1-6). Gastric and duodenal ulcerations are the most widely studied manifestations of injury to the gastrointestinal tract caused by NSAIDs, but these medications also can affect the jejunum and ileum, and can cause other types of abnormalities (inflammation, stricture, perforation, mucosal diaphragms and villous atrophy) (7,8).
Most of the previously available knowledge about the small bowel comes from autopsy examinations (9), case reports (10) or case series (11). Some techniques have been devised to study the small bowel injury (111In-labeled leukocytes, 51Cr-EDTA, fecal calprotectin levels), but they are indirect and are not generally available to the clinician (8). Push enteroscopy provides good visualization of the small bowel mucosa, and it has been used to study NSAID enteropathy (12), but does not examine the entire gut, is time-consuming, cumbersome for the patient, and usually requires sedation (13). Therefore, the true prevalence, location and extent of NSAID enteropathy are still unclear.
Capsule endoscopy (CE) is a new diagnostic method that has recently made non-invasive digital imaging of the entire small bowel possible. This technique has been demonstrated as the first-line diagnostic tool for detecting small bowel pathologies (14-16). The aim of this study was to evaluate the type, frequency, and severity of small bowel mucosal injury after chronic NSAID intake, as assessed by CE, as well as to correlate the presence of gastroduodenal and intestinal damage in these patients.
Methods
Patients and design
This was an endoscopist-blind, controlled study. Entry criteria for the study group included patients, ages 18-70 years, with OA in chronic therapy with NSAIDs. Patients with OA who had used no NSAIDs within the last 6 months were used as control group. Criteria for exclusion were concomitant use of antisecretory drugs, misoprostol, corticosteroids or aspirin; history of delayed gastric emptying, swallowing disorders, alcoholism, and gastric or intestinal resection; lactancy or pregnancy; and any clinical conditions advising non-participation in the trial. Women with childbearing potential who were not using reliable contraception were also excluded.
Capsule endoscopy and a conventional upper gastrointestinal endoscopy (UGE) were performed to all patients in the study group. Safety was assessed by physical examination, laboratory tests, and observed adverse events.
The primary endpoints were: a) percentage of subjects with small bowel mucosal injury; and b) percentage of subjects with normal mucosa, minor or major enteropathy. The rate of patients in the study group with major small bowel mucosal injury according to the severity of gastroduodenal lesions assessed by UGE was a secondary endpoint.
Procedures lesion assessment criteria
A wireless-capsule video (PillCamTM Given Imaging®, Yoqneam, Israel) was used. The system and the procedure have been described in detail elsewhere (17). Two investigators (J.M.H. and A.C.A.), who were blinded to the subject group and UGE results, separately reviewed each of the procedures beginning with the time of passage through the pylorus to passage to the cecum. After the independent review was complete, the findings were reviewed in conference by both investigators to reach a consensus.
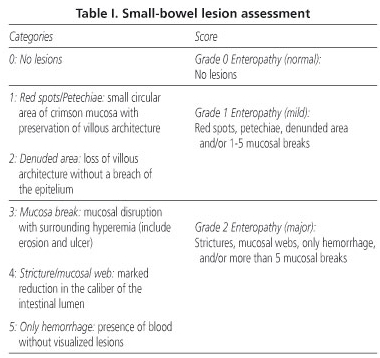
The descriptions of the findings were scored based on a self-designed scale where five categories were considered (Table I). The presence of red spots or petechiae, denuded areas and/or 1-5 mucosal breaks was considered as grade 1 enteropathy (minor), while the existence of more than 5 mucosal breaks and/or webs or strictures, or hemorrhage without visualized lesions was considered grade 2 enteropathy (major). Mucosal breaks included erosions and ulcers because the difference between both must be based on the size and depth of lesions and these parameters are difficult or impossible to assess with current capsule endoscopy.
CE does not allow for a good examination of the stomach so, in order to attempt a correlation between gastric and small bowel findings, a UGE was performed by an investigator (B.J.G-R., A.C.A., or F.J.P.) blinded to the assigned group at baseline and the results of CE. The presence and number of erosions and ulcers in the gastric or duodenal mucosa were assessed to classify patients as normal, grade 1 gastroduodenopathy (minor) or grade 2 gastroduodenopathy (major) (Table II).
Statistical analysis and ethics
Descriptive statistics were used for subject demographic and clinical characteristics. A statistical analysis was performed using chi square or Fisher's exact test with 2-tailed p value, whichever was appropriate, using SPSS software (version 10.0). For all the comparisons, statistical significance was set at p < 0.05. The clinical trial was conducted according to the Declaration of Helsinki, Good Clinical Practice (GCP) and local regulation. Both the patients and the healthy volunteers gave their written informed consent to participate in the clinical trial, which was approved by the Independent Ethics Committee.
Results
Thirty-three patients with OA were included, 23 women and 10 men, ages ranging from 31 to 69 years. Sixteen patients in chronic therapy with NSAIDs (study group) were compared to seventeen patients not receiving NSAID drugs (control group). Mean age was 57.47 ± 9.82 years for controls vs. 57.06 ± 10.60 for NSAID users (p = 0.91). Clinical and laboratory data for included patients are provided in table III.
Capsule endoscopy found intestinal lesions in 12 out of 16 patients (75%) in the study group and in 2 out of 17 volunteers (11.76%) in the control group (p < 0.0002; OR 22.20). Injury was mild in 5 out of 16 (31.5%) NSAID consumers as compared with 2 out of 17 controls (p = NS). Seven out of 16 NSAID consumers (43.75%) and none in the control group (0%) had major (grade 2) small bowel injury (p < 0.05) (Fig. 1). The most frequent intestinal damage among the study group were mucosal breaks (12 patients); denuded areas and erythema were also found in 7 and 3 patients, respectively (Fig. 2). In the control group, erythema and denuded areas were seen in 2 patients (Fig. 3).
The percentage of patients with grade 0, 1 and 2 gastroduodenopathy in the study group, as assessed by UGE, were 18.75% (3/16), 62.5% (10/16) and 18.75% (3/16), respectively. There was no significant difference in the rate of patients with grade 2 enteropathy beyween patients with grade 0-1 gastroduodenopathy (43.75%, 7/16) and those patients with grade 2 gastroduodenopathy (40%, 2/5; p = 0.65) (Fig. 4). There were no adverse effects caused by capsule endoscopy.
Discussion
The diagnosis of NSAID enteropathy is difficult, and most of the previously available knowledge about the small bowel comes from autopsy or scintigraphy examinations. In an autopsy study of 713 individuals, Allison et al. (9) found small bowel mucosal injury in 8.43% of 249 individuals who took NSAIDs within 6 months of their death, as compared to only 0.65% in the other 464 individuals (p < 0.01). Several studies have shown that long-term NSAID treatment is associated with enhanced migration of 111-Indium-labelled leukocytes to the ileum and increased fecal calprotectine shedding (18,19). These data add growing evidence that NSAIDs produce clinically significant inflammation with secondary hemorrhage and protein loss. Morris et al. (20) used probe-enteroscopy in 46 patients who were taking NSAIDs and had chronic iron-deficiency anemia with normal findings from conventional endoscopy investigations. In 41.3% of patients, enteroscopy showed intestinal erosions and ulcers.
However, several studies have demonstrated a higher prevalence of small bowel mucosal injury among patients with short or long-term NSAID intake when they were assessed by CE. Graham et al. (21) compared twenty-one rheumatic patients with chronic intake of NSAIDs and twenty patients who took only acetaminophen or nothing. Small bowel mucosal injury was seen in 71.43% of NSAID consumers and in 10% of controls (15/21 vs. 2/20; p < 0.01). The results of our series are almost identical to those reported by Graham et al. in group patients with similar characteristics. Maiden et al. (22) used CE to quantify and assess the nature of the small bowel mucosal injury caused by NSAIDs when taken on a short-term basis (14 days) in forty healthy volunteers and correlate the findings with those of the fecal calprotectin test. This study provides direct evidence of small bowel mucosal injury in 67.5% of volunteers resulting from 2 weeks' ingestion of slow-release diclofenac (75 mg twice a day), with more than one third having discrete mucosal breaks. In addition, thirty subjects (75%) had increased repeat fecal calprotectin concentrations above the upper limit of normal. The authors speculate about the possibility that an NSAID-induced increase in permeability leads to inflammation that is manifested sequentially as reddened, edematous folds, followed by areas of denuded villi, petechiae, erosions, and ulcers. However, in this study there was no significant correlation between fecal calprotectin and capsule endoscopy results. The reasons for these apparent discrepancies remain unclear.
The secondary endpoint of our study was to determine the rate of patients in the study group with small bowel mucosal injury according to the presence or absence of gastric or duodenal lesions as assessed by UGE. Our data showed that there was no higher frequency of major small bowel mucosal injury in those patients with moderate-severe gastroduodenal injury as demonstrated with UGE, so this could not be considered a predictive factor for macroscopic NSAID-enteropathy. Anyway, further trials are needed before drawing clinical or pathogenic conclusions on this topic.
Two critical issues can be argued when interpreting our results. First, although CE seems to be superior to other small bowel techniques in detecting NSAID mucosal injury and it could probably be considered the procedure of choice for the diagnosis of this disease, it is still an emerging technique for assessing small NSAID enteropathy. CE does not allow to determine the size and depth of lesions, and it is impossible to distinguish large erosions from small ulcers (21). Moreover, several score systems have been used to assess the small bowel mucosal injury caused by NSAID (22-24), what makes difficult the comparison of results from different series. It would be worthwhile to design a consensus score that can be validated in large, multicenter trials. Secondly, it is still unclear whether the findings seen on CE are clinically relevant in causing symptoms or having the potential for inducing gastrointestinal bleeding, perforation, anemia or hypoalbuminemia.
In summary, chronic NSAID use is associated with a high rate of macroscopic small bowel mucosal injuries. This damage can be detected by capsule endoscopy, which demonstrated to be a very useful tool for the diagnosis and assessment of NSAID enteropathy. Our data have failed to demonstrate a relationship between the severity of gastroduodenal and intestinal injury.
References
1. Cullen DJ, Seager JM, Holmes S, Doherty M, Wilson JV, Garrud P, et al. Pharmacoepidemiology of nonsteroidal anti-inflammatory drug use in Nottingham general practices. Aliment Pharmacol Ther 2000; 14: 177-85. [ Links ]
2. Anonymous. Retail and provider prospective: National prescription audit. Plymouth Meeting, PA, IMS Health; 2000. [ Links ]
3. Patrono C. Aspirin as an antiplatelet drug. N Engl J Med 1994, 330: 1287-94. [ Links ]
4. Husain SS, Szabo IL, Tarnawski AS. NSAID Inhibition of GI cancer growth: clinical implications and molecular mechanisms of action. Am J Gastroenterol 2002; 97: 542-53. [ Links ]
5. Straus WL, Ofman JJ. Gastrointestinal toxicity associated with nonsteroidal anti-inflammatory drugs: epidemiologic and economic issues. Gastroenterol Clin N Am 2001; 30: 895-920. [ Links ]
6. Lanas A. Cost stratification of nonsteroidal anti-inflammatory drug-associated gastrointestinal side effects. Med Clin (Barc) 2000; 114(Supl. 3): 46-53. [ Links ]
7. Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993; 104: 1832-47. [ Links ]
8. Fortun PJ, Hawkey CJ. Nonsteroidal anti-inflammatory drugs and the small intestine. Curr Opin Gastroenterol 2005; 21: 169-75. [ Links ]
9. Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated the use of non-steroidal anti-inflammatory drugs. N Engl J Med 1992; 327: 749-54. [ Links ]
10. Kwo PY, Tremaine WJ. Nonsteroidal anti-inflammatory drug-induced enteropathy: case discussion and review of the literature. Mayo Clin Proc 1995; 70: 55-61. [ Links ]
11. Lang J, Price AB, Levi AJ, Burke M, Gumpel JM, Bjarnason I. Diaphragm disease: pathology of disease of the small intestine induced by non-steroidal anti-inflammatory drugs. J Clin Pathol 1988; 41: 516-26. [ Links ]
12. Morris AJ, Madhok R, Sturrok RD, Capell HA, MacKenzie JF. Enteroscopic diagnosis of small bowel ulceration in patients receiving non-steroidal anti-inflammatory drugs. Lancet 1991; 337: 520-4. [ Links ]
13. Hayat M, Axon ATR, O'Mahony S. Diagnostic yield and effect on clinical outcomes of push enteroscopy in suspected small-bowel bleeding. Endoscopy 2000; 32: 369-72. [ Links ]
14. Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, et al. Clinical outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 2004; 126: 643-53. [ Links ]
15. Herrerías JM, Caunedo A, Rodríguez Téllez M, Pellicer FJ, Herrerías JM Jr. Capsule endoscopy in patients with suspected Crohn's disease in negative endoscopy. Endoscopy 2003; 35: 564-9. [ Links ]
16. Bowel disorders detection system approved. FDA news daily bulletin July 11, 2003. Available at: www.fdanews.com/dailies/bulletin/1_134/news/15318-1.html. Accessed September 7, 2005 [ Links ]
17. Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature 2000; 405: 417. [ Links ]
18. Bjarnason I, Fehilly B, Smethurst P, Menzies IS, Levi AJ. Effects of nonsteroidal antiinflammatory drugs on permeability of the small intestine in humans. J Rheumatol 1992; 19(Supl. 36): 83-4. [ Links ]
19. Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhold MK, Roseth A, et al. High prevalence of NSAID enteropathy as shown by a simple fecal faecal test. Gut 1999; 45: 362-6. [ Links ]
20. Morris AJ, Wasson LA, Mackenzie JF. Small bowel enteroscopy in undiagnosed gastrointestinal blood loss. Gut 1992; 33: 887-9. [ Links ]
21. Velayos B, Muñoz MF, Fernández L, Aller R, Lozano F, de la Calle F, et al. Measurement of lesion size by capsule endoscopy: an unsolved issue. Rev Esp Enferm Dig 2008; 100: 248-9. [ Links ]
22. Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol 2005; 3: 55-9. [ Links ]
23. Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005; 128: 1172-8. [ Links ]
24. Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazol and placebo. Clin Gastroenterol Hepatol 2005; 3: 133-41. [ Links ]
![]() Correspondence:
Correspondence:
Ángel Caunedo Álvarez.
Servicio de Gastroenterología.
Hospital Universitario Virgen Macarena.
Avda. Dr. Fedriani, s/n. 41071
Sevilla, Spain.
e-mail: acaunedoa@meditex.es - jmhg@us.es
Received: 16-09-09.
Accepted: 21-10-09.