Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.102 no.3 Madrid mar. 2010
Prognostic value of lymph node micrometastases in patients with colorectal cancer in Dukes stages A and B (T1-T4, N0, M0)
Valor pronóstico de las micrometástasis linfoganglionares en pacientes con cáncer colorrectal en estadios A y B de Dukes (T1-T4, N0, M0)
R. Uribarrena-Amezaga1, J. Ortego2, J. Fuentes1, N. Raventós3, P. Parra2 and R. Uribarrena-Echevarría1
1 Service of Digestive Diseases. Hospital Miguel Servet. Zaragoza, Spain.
2 Department of Pathology. Hospital Clínico Universitario Lozano Blesa. Zaragoza, Spain.
3 Service or Emergency. Hospital Miguel Servet. Zaragoza, Spain
ABSTRACT
Background: 30% of patients with colorectal cancer (CRC) in Dukes stages A and B (T1-T4, N0, M0) present tumor recurrence and die after 5 years follow up. This unexpectedly poor evolution might be attributable to the presence of lymph node micrometastasis undetected in routine examination with haematoxilin-eosine (H&E).
Objective: to assess the presence of undetected micrometastasis.
Patients and methods: we conducted a retrospective study of the locoregional lymph nodes in 85 patients operated for CRC in Dukes stages A and B (T1-T4, N0, M0), using immunohistochemistry with anticytokeratin antibodies AE1/AE3. In this descriptive, inferential bivariant and survival study, we analyzed different risk factors, including local infiltration T1/T4, Dukes A/B, number of dissected lymph nodes, vascular invasion, micrometastasis, tumor recurrence and death in the context of the presence or absence of micrometastases.
Results: Dukes stage and neoplastic angioinvasion are influential in patient prognosis; however, lymph node micrometastases were not associated with a poorer outcome of CRC.
Conclusions: locorregional lymph node micrometastases detected with anticytokeratine antibodies AE1/AE3 in Dukes A and B CRC patients are not associated with reduced survival.
Key words: Colorectal adenocarcinoma. Prognosis. Micrometastases.
RESUMEN
Introducción: un 30% de los pacientes con cáncer colorrectal (CCR) en estadios A y B de Dukes (T1-T4, N0, M0) presentan recidiva tumoral y/o fallecen a los 5 años. Esta inesperada mala evolución, en casos presumiblemente curados podría deberse, entre otras causas, a la presencia de micrometástasis linfoganglionares no detectadas en el estudio de rutina: hematoxilina-eosina (H&E).
Objetivo: determinar si la presencia de micrometástasis linfoganglionares detectadas mediante inmunohistoquímica con anticuerpos anticitoqueratina AE1/AE3, influyen en la evolución del CCR.
Pacientes y métodos: se han estudiado los ganglios linfáticos locorregionales de 85 pacientes con CCR en estadios A y B de Dukes (T1-T4, N0, M0), mediante técnicas de inmunohistoquímica con anticuerpos anticitoqueratinas AE1/AE3, para poner de manifiesto la presencia de micrometástasis. Se ha realizado un estudio descriptivo, inferencial bivariante y de supervivencia, según distintos factores de riesgo, centrado en la presencia o no de micrometástasis.
Resultados: hemos observado que el estadio de Dukes y la angioinvasión neoplásica son factores que influyen en el pronóstico de estos pacientes. Sin embargo, no se ha demostrado que la presencia de micrometástasis linfoganglionares se asocie a una peor evolución en el CCR.
Conclusiones: las micrometástasis linfoganglionares locorregionales detectadas mediante anticuerpos anticitoqueratina AE1/AE3, en pacientes con CCR en estadios A y B de Dukes, no se asocian a una menor supervivencia.
Palabras clave: Adenocarcinoma colorrectal. Pronóstico. Micrometástasis.
Introduction
Global 5 year survival in colorectal cancer (CRC) is approximately 50% and has not improved substantially in recent years, despite advances in diagnostic and therapeutic methods (1). Survival ranges between 80-90% in Dukes A, 60-80% in Dukes B, 30-35% in Dukes C, and 5-25% in D (2-5). The main prognostic factor in CRC is the presence or absence of metastasis in the regional lymph nodes (1,6).
Paradoxically, between 20 and 33% of patients diagnosed with CRC who are lymph-node negative (N0) based on conventional histological techniques experience tumor recidivism/death before 5 years (4,5). Thus, it is possible that a significant portion of Dukes A and B tumors are miscategorized because of missed metastases.
There are two problems with the usual technique of lymph node dissection, which involves sectioning every node into parts and selecting one for paraffin embedding, sectioning and hematoxilyn-eosin staining: so only part of the lymph node tissue is examined, and the customary staining with H&E may not detect small accumulations of tumor cells (7,8). Therefore, the proposed miscategorization might arise from the low sensitivity of conventional histological techniques in detecting lymph node micrometastases.
Micrometastases are small deposits of tumor cells less than 2 mm in diameter, in the regional lymph nodes. They are distinct from macrometastases because they do not have their own blood supply and they take in nutrients and oxygen by passive diffusion, which limits their growth. These small groups of tumors may remain dormant for long periods until the immune system eliminates them or until angiogenesis allows formation of new blood vessels that permit their growth (8).
To detect micrometastases, diagnostic techniques more sensitive than H&E are required, such as immunohistochemistry or polymerase chain reaction (PCR) (8,9). Most immunohistochemical techniques for detecting CRC use anticytokeratin monoclonal antibodies. These antibodies reveal the presence of epithelial cells in the lymph node, indicating their neoplastic metastatic nature (8,10). The micrometastases may also be detected with antibodies to carcinoembryonic antigen (CEA), a protein that is overexpressed in 96% of CRCs and not present in normal cells (11), and with antibodies to β-HCG, which is greatly increased in tumor cells of the gastrointestinal tract (12). In 19 to 32% of the negative lymph nodes in H&E studies, micrometastases have been found using immunohistochemical methods (13-16). This fact, combined with the recurrence in patients with N0 tumors led us to investigate the role of micrometastases in the evolution of CRC.
Patients and methods
Patients
A retrospective study was performed of 85 patients diagnosed with and treated for Dukes stages A and B CRC (N0, M0) at the Hospital Clínico Universitario Lozano Blesa in Zaragoza (HCU) and the Hospital Provincial de Zaragoza (HPZ) between years 1995-2000. Exclusion criteria were as follows: a) the adjuvant administration of radiotherapy and chemotherapy; b) the existence of lymph node metastases detectable using conventional histological techniques (H&E); c) the presence of distant metastasis at the moment of surgery; and d) the occurrence within the 5 years before or after the CRC diagnosis of other neoplasias that might influence in the course of CRC in the patients.
Immunohistochemical analysis
For every case of CRC, we identified the associated blocks of tissue, which had been fixed in 10% neutral formalin and embedded in paraffin.All blocks corresponding to lymph nodes were selected and cut in 5 μm thick sections that were deparaffinized, hydrated, and transferred to citrate buffer (pH 6). Afterwards, endogenous peroxidase activity was blocked with incubation in 5% H2O2 in methanol, followed by antigen retrieval with pressure cooking in citrate for 10 minutes (Target Retrieval Solution, Dakocytomation). Sections were transferred to blocking serum (horse serum) and then exposed to wide spectrum Ac anticytokeratins (AE1/AE3, Dakocytomation, Glostrup, Denmark) in a 1/500 dilution to detect the presence of epithelial cells in the lymph nodes (Figs.1-3).
The detection method used was the EnVision kit (from Dakocytomation, Glostrup, Denmark), with diaminobenzidine as the developer and hemotoxylin as the chromogen.
Statistical analysis
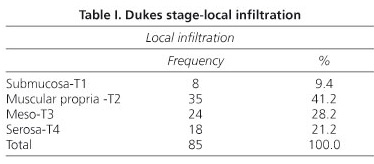
We used descriptive statistics to analyze tumor characteristics, which included local infiltration (submucosa, T1; muscle, T2; meso, T3; and serosa, T4); Dukes stages (A or B; C and D were excluded); number of lymph nodes dissected; presence or absence of vascular, perineural, or lymph node invasion; presence or absence of micrometastasis; presence or absence of tumor recurrence and month of the recurrence; mortality (if applicable); and month of death (if applicable).
A bivariant inferential study was also performed to identify differences, if any, between patients who presented micrometastases and those who did not in relation to a series of variables included in the study. To this end, Pearson's Chi-squared test was applied, with the Yates correction or Fisher's exact test if needed. The Student t-test, analysis of variance (ANOVA), the non-parametric Mann-Whitney U test, and the Kruskal-Wallis test were also used. In cases where the ANOVA or the Kruskal-Wallis test identified significance, multiple comparisons were performed between the groups to ascertain those that were significantly different. These comparisons were made by applying the Pearson correlation coefficient and the Spearman rank coefficient. The confidence level chosen for the tests was 95%.
Finally, survival was analyzed by means of the Mantel-Henzel technique.
Results
Of the 85 cases studied, 42 were Dukes A and 43 Dukes B. According to local infiltration, 8 patients were T1, 35 were T2, 24 were T3, and 18 were T4 (Table I). Twenty-three were localized in the rectum, 54 in sigmoid colon and 8 in transverse and right colon. Mean age was 67.32 years with a range of 29-88 years. 49 of the patients were male and 36 female.
An average of 10.75 lymphatic nodes were dissected per patient (standard deviation 6.72; mean confidence level 9.3-12.2). Micrometastases were detected in 31 cases (36.5%).
Recurrence of the disease and later death due the tumor occurred in 22 cases (26.9%). On average, recurrence took place in month 23.73 of follow-up (range 2 months to 82 months; standard deviation 20.24; mean confidence level 14.75-32.7).
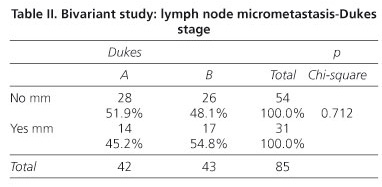
In the bivariant inferential study patients were divided into two groups for this study, those with no micrometastases and those in whom micrometastases were identified.
Of the 54 patients in whom micrometastases were not detected (the micrometastases-negative group), 28 (51.9%) were Dukes A and 26 (48%) Dukes B. Of the 31 patients with lymph node micrometastases (the micrometastases group), 14 (45.2%) were Dukes A and 17 (54.8%) Dukes B. A calculated Pearson Chi-squared of 0.712 (> 0.05) established no statistical association between the presence of lymph node micrometastases and Dukes stage (Table II).
However, there was a correlation between the presence of micrometastases and the number of lymph nodes dissected. In the micrometastases group, an average of 15 lymph nodes was dissected compared to 8.31 in the micrometastases-negative group. With a p value = 0.000 (< 0.05) given by the Mann-Whitney U test, the micrometastases group presented a significantly greater number of dissected lymph nodes.
Both groups were also compared for tumor recurrence and death. In the micrometastases-negative group, 12 patients (22.2%) suffered tumor recurrence leading to death, compared to 10 patients (32.3%) in the micrometastases group. However, this difference, with a Pearson Chi Squared test p value of 0.477, was not statistically significant. Thus, there was no association detected between micrometastasis, tumor recidivism, and death (Table III).
A Kaplan-Meier survival study was performed to determine the prognostic factors in CRC. First of all, we studied the Dukes stage. In the Dukes A group (42 cases), six died from recurrence of the disease (14.29%). In the Dukes B group (43 cases), 16 patients (37.21%) suffered tumor recurrence that led to death.
In the survival curve graph shown in figure 4, the Kaplan-Meier product-limit calculation for Dukes A-B shows that there are differences between the groups in terms of recurrence. The Mantel-Haenszel contrast (log-rank) showed that people with Dukes B present a significantly higher risk of CRC recurrence and death (statistic = 6.78: p value = 0.0092) (Fig. 4). If we analyze local invasion, survival rate in T1 was 87.5%, in T2 82.86%, in T3 62.5% and in T4 66.67%. T1 and T2 patients had a significantly better prognosis than T3 and T4 colorectal cancers (statistic = 4.03, p value = 0.0446).
We also analyzed as a prognostic factor vascular invasion by neoplastic cells. Vascular invasion was detected in only 3 of the 85 patients studied, and two of these patients experienced CRC recurrence (66%). Of the remaining 82 cases, recurrence was observed in 20 (24.39%).
The Kaplan-Meier product-limit calculation for vascular invasion (no, yes) identified differences between the groups in terms of recurrence. To extrapolate this conclusion to the population level, we used the Mantel-Haenszel (log-rank) contrast and found that patients with vascular invasion presented a significantly higher risk of recurrence and death due to the tumor (statistic = 4.94; p value = 0.0262).
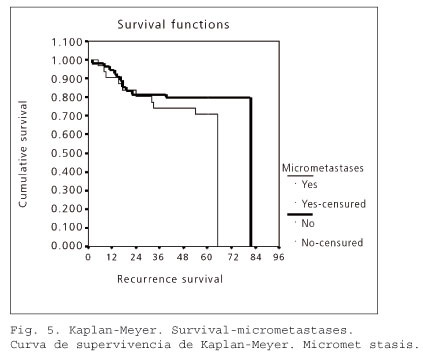
Finally, we analyzed the prognostic impact of lymph node micrometastasis. In the micrometastases-negative group (54 cases), 12 patients (22.2%) experienced tumor recurrence. In the micrometastasis group (31 patients), recurrence occurred in 10 cases (32.26%).
In the survival curve graph shown in table V, the Kaplan-Meier product-limit calculation for lymph node micrometastases (no, yes) found no differences between the groups with regard to recurrence. The Mantel-Haenszel contrast (log-rank) showed no significant differences between the two groups (statistic = 1.11; p value = 0.2916). Thus, the presence of micrometastasis was not associated with a worse prognosis in CRC (Fig. 5).
Discussion
As other authors have reported, the main factor influencing prognosis in CRC is the presence or absence of metastasis in the regional lymph nodes. The main decrease in survival occurs between Dukes stages B (N0) and C (N1) (1,6).
It is notable that approximately 30% of patients with CRC who show no sign of lymph node involvement on routine histological evaluation die from recurrence of the disease (2-5). In our study, 12.5% of the patients with Dukes A CRC and 37.8% of those with Dukes B suffered tumor recurrence leading to death, reaching a global figure of 25.8% for the Dukes A and B groups.
The Dukes stage and the local growth of the tumors are prognostic factors that have been widely accepted in CRC evaluation (2-5,17,18). As expected, the survival analysis identified a significantly higher frequency of tumor recurrence in cases classified as Dukes B in comparison to Dukes A.
We also found a statistically significant relationship between the existence of vascular invasion and the evolution of the disease. The finding of small isolated accumulations of tumor cells in the blood vessels is associated with a meaningful increase in tumor recurrence, with a subsequent decrease in survival rates. Tumor angioinvasion is a necessary step for the formation of remote metastasis, and this finding coincides with the majority of studies on revised prognostic factors (19-22). However, the low number of cases with vascular invasion devalues this finding.
The influence of lymph node micrometastases only visible by means of immunohistochemistry in the prognosis of CRC is a controversial topic. Previous studies have reported varied results. As we have verified, the greatest drop in CRC survival can be seen from Dukes B to C. If the existence of micrometastases were as significant as conventional lymph node metastasis, the difference in prognosis between patients with and without micrometastases should be clear.
A mean of 10.75 lymph nodes were resected. First, they were cut again and restained with H&E. Two N0 cases were upstaged to N1 (Dukes C) because of the detection of macrometastases in this second look and were excluded. Secondly we studied the lymph nodes with immunohistochemical techniques. One of the advantages of immunohistochemistry is the possibility of using tissue samples preserved in paraffin, which permits retrospective studies, as in our case (23).
Micrometastases were found in 36.5% of our cases that had been classified by H&E as unaffected. In other studies, micrometastases were detected in 32% of the cases diagnosed as N0 by H&E (24); thus, our micrometastases detection percentage is slightly higher. The study by Tschmelitsch et al. (25) is an extreme example: they detected micrometastases in 76% of the lymph nodes that had been classified as negative based on H&E and explain this discrepancy as arising from the high number of lymph nodes studied in each case (an average of 16). Other authors have used monoclonal antibodies targeting different proteins such as CEA, cytokeratin 20 (CK 20), and cytokeratins AE1/AE3 (11,14,25-29), which might result in variability in the tumor-cell-detection sensitivity. A large review made by Nicastri et al. (9), measured the different sensibility of the several antibodies used in immunohistochemistry for micrometastases detection and pointed out the lack of a "gold standard". AE1/AE3 was most widely used antibody in reviewed articles, the same marker we used in our study. Kong et al. (30) used quantitative real time PCR to detect biomarkers like CEA CK 20 and guanylyl cyclase C in the detection of lymph node micrometastases. They also agreed in the variability for the different antigens in the detection of micrometastases and concluded that the combination of different biomarkers could increase the sensibility of this technique.
In the bivariant study, we found significant differences between the existence of micrometastases and the number of lymph nodes dissected. In the micrometastasis cases, a greater number of lymph nodes were dissected (an average of 15 lymph nodes) in comparison to the cases without micrometastases (an average of 8.3).
An explanation of this finding could be the immunological stimulation. The presence of the primary tumor and micrometastases could induce a lymph node formation/proliferation as a local immuno-response (31).
Of the 54 patients without micrometastasis, 12 suffered tumor recurrence leading to death (22.2%). A total of 10 of the 31 cases with lymph node micrometastasis resulted in death (32.25%). However, this 10% increase of recurrence and death in the micrometastasis group was not statistically significant. Therefore, although survival differences were observed, we cannot conclude that lymph node micrometastasis is a predictive factor of tumor recurrence or of an increased risk of death due to CRC. This agrees with two recent studies published by Steinert et al. (32) and Fleming et al. (33). Nicastri et al. (9), in their review, could not conclude that micrometastases detected by immunohistochemical techniques with a clinically worse outcome. They attribute the different results of these studies to the diverse methodology and the different antibodies used. In the same review they conclude that most of the articles that used RT-PCR for micrometastases were able to find clinical relevance.
In conclusion, the results suggest that locorregional lymph node micrometastases detected with anticytokeratine antibodies AE1/AE3 in Dukes A and B CRC patients are not associated with reduced survival.
Acknowledgments
The authors would like to thank Lidia Floria, Carmen Marcellán, Pilar Pina, Sara Serrano, and Carolina Villalba, Superior Technicians of Pathological Anatomy, for their collaboration, and Dr. Javier Mateos, of the Service of Pathology of the Hospital Provincial Ntra. Sra. de Gracia, Zaragoza, for the contribution of several cases.
References
1. Fielding P. Staging Systems. In: Cohen A, Vimawer S, editors. Cancer of the colon, rectum and anus. New York: McGraw-Hill; 1995. p. 207. [ Links ]
2. Itzkowitz SH. Cáncer gastrointestinal: cáncer de colon y recto. In: Wilcox CM, editor. Digestive diseases self education program. Barcelona: Medical Trends; 2001. p. 25. [ Links ]
3. Hermanek P. pTNM and residual tumor classifications: problems of assesment and prognostic significance. World J Surg 1995; 19: 180-90. [ Links ]
4. Bilchik AJ, Saha S, Wiese D, Stonecypher JA, Wood TF, Sostrin S, et al. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol 2001; 19(4): 1128-36. [ Links ]
5. Ovaska J, Järvinen H, Kujari H, Pertilä I, Mecklin JP. Follow up of patients operated on for colorectal carcinoma. Am J Surg 1990; 159: 593-6. [ Links ]
6. Newland RC, Chapuis PH, Pheils MT, MacPherson JG. The relationships of survival to staging and grading colorectal carcinoma: a prospective study of 503 cases. Cancer 1981; 47: 1424-9. [ Links ]
7. Bilchik AJ, Nora D, Tollenar RAEM, Van de Velde CJH, Wood T, Turner R, et al. Ultrastaging of early colon cancer using lymphatic mapping and molecular analysis. Eur J Cancer 2002; 38(7): 977-85. [ Links ]
8. Kell MR, Winter DC, O'Sullivan GC, Shanahan F, Redmond HP. Biological behaviour and clinical implications of micrometastases. Br J Surg 2000; 87(12): 1629-39. [ Links ]
9. Nicastri DJ, Doucette JT, Godfrey TE, Hugues SJ. Is occult lymph node disease in colorectal cancer clinically significant? A review of the relevant literature. J Mol Diagn 2007; 9(5): 563-71. [ Links ]
10. Moll R, Franke WW, Schiller DI, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31: 11-24. [ Links ]
11. Cutait R, Alves VA, Cámara L, Cutait DE, Borges JL, Singer J, et al. Restaging colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum 1991; 34: 917-20. [ Links ]
12. Wong JH, Steineman S, Caldería C, Bowles J, Namiki T. Ex vivo sentinel node mapping in carcinoma of the colon and rectum. An Surg 2001; 233(4): 515-21. [ Links ]
13. Greeson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW. Identification of occult micrometastases in pericolic lymph nodes of Dukes'B colorrectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Cancer 1994; 73(3): 563-9. [ Links ]
14. Liefers GJ, Cleton-Jansen AM, Van de Velde CJ, Hermans J, Van Krieken HJM, Cees J, et al. Micrometastases and survival in stage II colorectal cancer. N Eng J Med 1998; 339(4): 223-8. [ Links ]
15. Bendavid Y, Latulippe JF, Younan RJ, Leclerc YE, Dube S, Heyen F, et al. Phase I on sentynel lymph node mapping in colon cancer: A preliminary report. J Surg Oncol 2002; 79(2): 81-4. [ Links ]
16. Shimoyama M, Yamazaki T, Suda T, Hatakeyama K. Prognostic significance of lateral lymph node micrometastases in lower rectal cancer: an immunohistochemical study with CAM5.2. Dis Colon Rectum 2003; 46(3): 333-9. [ Links ]
17. Bouvet M, Milas M, Giacco GG, Cleary KR. Janjan NA, Skibber JM. Predictors of recurrence after local excision and postoperative chemoradiation therapy of adenocarcinoma of the rectum. An Surg Oncol 1999; 6(1): 26-32. [ Links ]
18. Di Gregorio C, Benati P, Losi L, Roncucci L, Pedroni M, Scarselli A, et al. Incidence and survival of patients with Dukes A (Stage T1 and T2) in colorrectal carcinoma: A 15-year population based study. Int Colorectal Dis 2005; 20(2): 147-54. [ Links ]
19. Offerhaus GJ, Giardiello FM, Bruijn JA, Stijnen T, Molyvas EN, Fleuren GJ. The value of immunohistochemistry for collagen IV expression in colorectal carcinoma. Cancer 1991; 67(1): 99-105. [ Links ]
20. Celen O, Yildrim E, Berberoglu U. Factors influencing outcome of surgery for stage I rectal cancer. Neoplasma 2004; 51(6): 487-90. [ Links ]
21. Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled data analysis. Dis Colon Rectum 2005; 48(8): 1588-96. [ Links ]
22. Meguerditchian AN, Bairati I, Lagace R, Harel F, Kibrite A. Prognostic significance of limphovascular invasion in surgically cured rectal carcinoma. Am J Surg 2005; 189(6): 707-13. [ Links ]
23. Goldenberg DM, Sharkey RM, Primus FJ, Carcinoembryonic antigen in histopathology immunoperoxidase staining of conventional tissue sections. J Natl Cancer Inst 1976; 57: 11-22. [ Links ]
24. Oberg A, Stenling N, Tavelin B, Lindmark G. Are lymph node micrometastases of any clinical significance in Dukes A and B colorectal cancer? Dis Colon Rectum 1988; 41(10): 1244-9. [ Links ]
25. Tschmelitsch J, Klimstra DS, Cohen AM. Lymph node micrometastases do not predict relapse in stage II colon cancer. Ann Surg Oncol 2000; 7: 601-8. [ Links ]
26. Rosemberg R, Hoos A, Mueller J, Baier P, Stricker D, Werner M. Prognostic significance of cytokeratin-20 reverse trancriptase polymerase chain reaction in lymph nodes of node-negative colorectal cancer patients. J Clin Oncol 2002; 20(4): 1049-55. [ Links ]
27. Greeson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW. Identification of occult micrometastases in pericolic lymph nodes of Dukes' B colorrectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Cancer 1994; 73(3): 563-9. [ Links ]
28. Bilchik AJ, Hoon DS, Saha S, Turner RR, Wiese D, Di Niome M, et al. Prognostic impact of micrometastases in colon cancer: Inerim results of a prospective multicenter trial. Ann Surg 2007; 246: 568-75. [ Links ]
29. Park SJ, Lee KY, Kim SY. Clinical significance of lymph node micormetastases in stage I an II colorectal cancer. Cancer Res Treat 2008; 40(2): 75-80. [ Links ]
30. Kong SL, Salto-Téllez M, Leong AP, Chan YH, Koay ES. Discordant quantitative detection of putative biomarkers in nodal micrometastases of colorectal cancer: Biological and clinical implications. J Clin Pathol 2005; 58(8): 839-44. [ Links ]
31. Takeuchi H, Kitajima M, Kitagawa Y. Sentinel lymph node as a target of molecular diagnosis of lymphatic micrometastasis and local immunoresponse to malignant cells. Cancer Sci 2008; 99(3): 441-50. [ Links ]
32. Steinert R, Hantschick M, Vieth M, Gastinger I, Khünel F, Lieperth H, et al. Influence of subclinical tumor spreading on survival, after curative surgery for colorectal cancer. Arch Surg 2008; 143: 122-8. [ Links ]
33. Flemming FJ, Hayanga AJ, Glynn F, Thakore H, Kay E, Gillen P. Incidence and prognostic influence of lymph node micrometastases in rectal cancer. Eur J Surg Oncol 2007; 33: 998-1002. [ Links ]
![]() Correspondence:
Correspondence:
Rafael Uribarrena Amezaga.
Servicio de Enfermedades Digestivas.
Hospital Miguel Servet.
Paseo Isabel la Católica, 1-3. 50009 Zaragoza.
e-mail: uribarrenaa@hotmail.com
Received: 21-09-09.
Accepted: 11-11-09.











 texto en
texto en