Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.102 no.9 Madrid sep. 2010
Gastrointestinal carcinoid tumors
Tumores carcinoides digestivos
M. J. Varas Lorenzo, F. Muñoz Agel, J. C. Espinós Pérez and M. Bardají Bofill
Unit of Endoscopy. Centro Médico Teknon. Barcelona, Spain
ABSTRACT
Objective: carcinoid tumors (CTs) represent the commonest neuroendocrine tumors.
Those in the gastrointestinal tract are diagnosed in surgical specimens, clinically, and using imaging techniques (endoscopy, echoendoscopy, CT, Octreoscan, etc.).
The goal of this retrospective study was to review a personal series of gastrointestinal carcinoid tumors, and to compare it to those in the literature.
Patients and methods: the medical records of 40 Caucasian patients with over 50 gastrointestinal carcinoid tumors (including multiple cases) who were seen for a period of 16 years (1994-2009) were reviewed.
Results: mean age at presentation was 52 years, 50% were females, and mean tumor size was 9.9 mm. Most were gastroduodenal (42.5%) or rectal (30%), and were treated endoscopically. Metastases and carcinoid syndrome (CS) were seen in 5% of patients. Survival at study endpoint was 85%.
Conclusions: age and gender were consistent with the literature. There was an increase in gastroduodenal (multifocal) and rectal carcinoids, likely because the series was essentially endoscopical in nature (bias). There was a lower rate of CS and higher survival, likely due to earlier diagnosis and treatment.
Key words: Gastrointestinal carcinoid tumors. Neuroendocrine tumors. Carcinoid syndrome.
RESUMEN
Objetivo: los tumores carcinoides (TC) son los tumores neuroendocrinos más frecuentes. Los digestivos se diagnostican en las piezas quirúrgicas, en la clínica, y mediante los métodos de imagen (endoscopia, ecoendoscopia, TAC y Octreoscan, etc.).
El objetivo de este trabajo retrospectivo fue revisar una serie personal de tumores carcinoides digestivos y compararla con la literatura.
Pacientes y métodos: se revisaron las historias clínicas de 40 pacientes de raza blanca con más de 50 tumores carcinoides digestivos, algunos múltiples, observados durante 16 años (1994-2009).
Resultados: la edad media de presentación fue 52 años, 50% mujeres, con un tamaño medio del tumor de 9,9 mm. La mayoría eran gastroduodenales (42,5%) y rectales (30%) y fueron tratados por vía endoscópica. Las metástasis y el síndrome carcinoide (SC) se observó en un 5% de los casos. La supervivencia en el momento de cerrar el estudio era del 85%.
Conclusiones: la edad y el sexo fueron similares a lo descrito en la literatura. Hubo un aumento de los carcinoides gastroduodenales (multifocales) y rectales, probablemente porque la serie era fundamentalmente endoscópica (sesgo). Se observó una disminución de la aparición SC y un aumento de la supervivencia probablemente por un diagnóstico y tratamiento más precoz.
Palabras clave: Tumores carcinoides digestivos. Tumores neuroendocrinos. Síndrome carcinoide.
Introduction
Carcinoid tumors (CTs) were described more than 100 years ago by Oberndorfer (1,2). MacDonald (1) found 0.02% carcinoids in surgical cases, and 1% in necropsies.
The incidence of carcinoid tumors has increased over time from 0.32/100,000 population and year (3) in the only community-based study available to 1-2 cases/100,000/year (4,5), and then further to 4.4/ 100,000/year (6,7); the rate in surgical specimens and necropsies was 8.4/100,000/year in Sweden during a 12-year period of time (8). The one study in our country (9) points out an incidence of 0.7/100,000 population/year for all CTs (0.125% in necropsies).
CTs occur twice as much in Afro-American than in Caucasian patients (10). Gender distribution is similar, slightly higher in females for malignant carcinoids at any age, but predominating in the sixth decade of life.
Gastrointestinal carcinoids used to predominate (74%) over bronchopulmonary ones (25%), 67.5 versus 25.3% (6) (Table I), but this proportion has been inverted of late (20 versus 72%) (11). Differences are due to the varying series and studies: clinical, surgical, or necropsy-derived.
Appendicular (60%) (1,9), rectal, and ileal (4,12) CTs were most common. Intestinal CTs represent 1% of gastrointestinal tract malignancies.
Another register shows an increase in pulmonary, gastric, and intestinal CTs, and a decrease in appendicular CTs (5).
Reviews have been performed (12) and series have been reported for gastric, duodenal, intestinal, appendicular, rectal, etc., carcinoids.
These tumors may give rise to a typical carcinoid syndrome (CS) (flushing, bronchoconstriction and watery diarrhea) or an atypical one (CS-like).
Objective
The goal of this retrospective study was to review a personal series of gastrointestinal carcinoid tumors, and to compare it to an updated review on this topic.
Patients and methods
Forty Caucasian patients with over 50 gastrointestinal carcinoid tumors seen during 16 years were reviewed (1994-2009); several subjects had multifocal tumors and one was a multiple site case: a patient with MEN-1 had several gastric and one retroperitoneal carcinoids. Only one had a typical carcinoid syndrome, and one had an atypical carcinoid syndrome.
Bias: the series was mainly based on endoscopy and echoendoscopy (EUS) cases, therefore the number of gastroduodenal and colorectal carcinoids is higher than that of appendicular (appendicitis) or intestinal (subocclusion) ones, whose frequency is higher in surgical series.
Among all 40 patients, 25 gastroduodenal and colorectal tumors were treated endoscopically, and 16 patients with 21 CTs underwent EUS-assisted endoscopical resection once metastatic disease had been ruled out using CT scans and Octreoscan.
Demographic parameters (race, age, sex, etc.), variables related to endoscopic and echoendoscopic tumor characteristics (size, morphology, etc.), lesion location, clinical manifestations, management, and survival at review were all analyzed.
The statistical analysis was carried out using the SPSS 11.5 for Windows software.
Results
Mean age at presentation was 52 years (range: 13-81 years). Sex: 20 M and 20 F (50%). All patients were Caucasian.
CT location: 0 esophagus, 14 stomach (35%), 3 duodenum (7.5%), 3 intestine (7.5 %), 5 appendix (12.5%), 1 colon (2.5%), 12 rectum (30 %), 1 pancreas, and 1 liver (2.5%).
Multifocal in one organ: 10 in stomach (25%), 1 in duodenum, 1 in intestine (2.5%).
Multiple sites: one case of MEN-1 with multifocal gastric tumors and one retroperitoneal carcinoid; it was managed with somatostatin analogues.
Mean size of lesions: 9.9 mm (range: 2-35 mm).
Endoscopy, echoendoscopy (EUS) and CT scans were the determinant diagnostic modalities.
Treatment: polypectomy (14 cases), band-assisted mucosectomy (6 cases), transanal endoscopic microsurgery (TEM) (2 cases), appendectomy (5 cases), surgical resection (3 cases), somatostatin (3 cases), and chemotherapy (2 cases). None was treated with growth factor inhibitors.
Endoscopic management was used for 25 gastroduodenal and colorectal tumors, and 16 patients with 21 CTs underwent EUS-assisted endoscopic resection. This series is the subject of a different report.
Metastases and CS: 2/40 (5%)
Survival: 34/40 (85%).
Discussion
Tumors in the esophagus (0.1%) (11), bile ducts, papilla, and pancreas are anecdotal findings (12) (Tables I and II).
One of our cases involved the pancreas and had liver metastases, had been surgically confirmed, and had a carcinoid-like syndrome that was treated with somatostatin analogues (13).
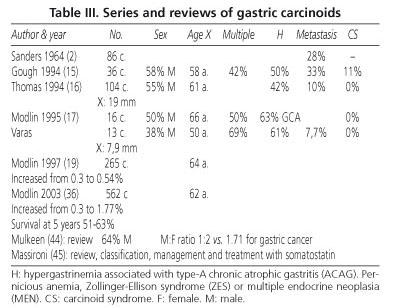
Gastric CTs amount to less than 1% of gastric neoplasms, and represented 2-4% of all carcinoids (4,5), but their rate has increased to almost 12%.
Up to more than 70% of cases (12,14-19) are associated with type-A chronic atrophic gastritis (ACAG) or pernicious anemia (gastric CTs, type 1). They are usually smaller than 1 cm and multifocal in 50%. Hypergastrinemia plays a fundamental role (14,15), so chronic therapy with Sandostatin is appropriate.
Around 5-10% of cases (gastric CTs, type 2) are associated with Zollinger-Ellison syndrome (ZES) in the setting of MEN-1 under genetic influence. Treatment and prognosis are similar to those associated with ACAG.
Approximately 15-25% of cases are sporadic (gastric CTs, type 3), single lesions greater than 1-2 cm, and originate metastases in over 50% of patients (Ki greater than 2%). They have been associated with an atypical carcinoid syndrome induced by histamine.
When greater than 2 cm these tumors are usually fatal (16). In our series no gastric CT was greater than 20 mm, and most were treated endoscopically (Table III).
Their rate and incidence have increased (19), which our series also seems to corroborate.
Duodenal CTs are usually small, less than 1 cm in size (our cases were equal to or smaller than 5 mm) and located in the bulb (20). In the most extensive series (21) with 24 cases, 89% were smaller than 2 cm, and 85% were confined to the mucosa and submucosa, with a survival rate of 100% at nearly 4 years.
Intestinal tumors represent 1% of gastrointestinal tract malignancies (22), with an incidence of 0.7 in males and 0.6/100,000 in women (22); they are usually located in the ileum and are multifocal or multisite, induce metastasis in lymph nodes and the liver (second only to right colon tumors in metastasis production), and result in typical carcinoid syndrome (flushing and watery diarrhea) because of serotonin (5-HIAA), substance P, etc., in 5-7% of patients.
Among the three cases in our series two were single lesions and one was multifocal (subocclusion); none had liver metastasis, and both responded well to surgery (Fig. 1).
They induce bleeding, mesenteric fibrosis, and occlusion leading to intestinal resection.
Malignant carcinoid tumors have a survival rate of 65% at 5 years (12). In a recent study of 3,911 intestinal carcinoids during 30 years (23) 5-year survival was 63%, 74% for localized CTs, 72% for CTs with regional metastasis, and 43% for those with distant metastases. This register claims that intestinal CTs are most common (21% of all gastrointestinal CTs), and their rate is increasing mainly in women and black patients.
Appendicular CTs are diagnosed in the fourth or fifth decade of life (12,24); in younger patients they are found in appendectomy specimens after acute appendicitis (25); they are more common in women than in men (25). Four of the 5 patients in our series were females.
Tumor size is the most significant prognosis factor -95% are smaller than 2 cm and appendectomy suffices. One third are greater than 2 cm and present with metastasis, which requires right colectomy (24,25).
Survival at 5 years is 94% when the disease is confined to the appendix (5), 85% when regional metastases exist, and 34% when distant metastases are present (even liver metastases, which may result in carcinoid syndrome).
Among gastrointestinal CTs appendicular tumors are most common, with rates of 44% (4), 60% (1), 66% (9), 78% (26), and 73% (27).
Colonic CTs are rare and big, represent less than 1% of all colonic tumors, and fewer than 5% induce CS. Five-year survival is 70% when local (5), 44% when regional metastases are present, and 20% for cases with distant metastases.
In our series only one colonic carcinoid was found, which was 30 mm in size and had no metastasis.
Rectal carcinoids represent 1.8% of rectal neoplasms (4) and up to 15-20% of gastrointestinal carcinoids (29,30), and are more common in the sixth decade of life (5). Around 50% are asymptomatic and represent chance findings during routine colonoscopies in the USA. Most common symptoms include anorectal complaints (29). Survival after 5 years is 81, 47, and 18%, respectively (5) in the USA. Survival was 85% in Japan (30).
Those smaller than 1 cm are treated with local excision because of their early diagnosis (30). Tumors greater than 2 cm represent surgical cases (low anterior resection or abdominoperineal resection depending on their level in the rectum), since these lesions have metastasis in 83% of cases and muscularis propria infiltration in 88% of patients (31).
The management and therapy of tumors 1-2 cm in size is controversial, but most will benefit from local treatment, with polypectomy versus band-assisted resection (32-34). Most of our cases were treated with polypectomy, band-assisted mucosectomy, and TEM.
In our series, likely because of its bias, gastric and rectal lesions were most common, and were managed with endoscopic therapy (polypectomy and bands, mainly) (35) (Fig. 2).
The incidence of gastrointestinal (23,36) and rectal CTs is increasing when compared to that seen 25 years ago, around 0.4/100,000/year (3,35); it is probably above 4 cases/100,000 population/year, whereas in the USA the incidence of bronchopulmonary CTs was 1.57/100,000 in 2003 (37). Total CT incidence is higher than 5 cases/100,000/year, and digestive lesions, mainly intestinal tumors, remain preponderant (38-41); the prevalence and incidence of rectal CTs (42,43) and gastric CTs (44-46) is also increasing. Polish authors (43) analyzed over 50,000 colonoscopies in a screening program for colorectal cancer, and found 25 carcinoids (prevalence: 0.05%) in 24 patients with a mean age of 54 years; maximum tumor size was 10 mm (mean: 6 mm). The increase in gastric CTs may possibly result from screening with gastroscopy and biopsies, as well as from a greater use of immunocytochemistry (positivity for chromogranin and synaptophysin is pathognomonic for carcinoid) (46).
The prognosis of CTs depends on their size (1 cm), the presence or absence of metastasis, and the development of CS (less than 10%). Tumors greater than 1 cm metastatize to the liver in 58% of cases, and show a 5-year survival rate of 43% in a classic series of 156 cases -47 appendicular (30%) and 41% multiples lesions (35).
The Brazilian registry (11) shows a 5-year survival rate around 70%, and 10-year survival at 50%; at the time of analysis 30% of patients were still alive, and 46% of these had no evidence of disease. Current survival in our series is 85%.
Survival for rectal and gastric CTs has increased over 20% in late reviews, probably due to earlier diagnosis (endoscopy, biopsies, echoendoscopy, CT scanning, and Octreoscan) and treatment (42,46).
Somatostatin analogues may also have probably represented an advance in the management of CTs, particularly of gastric tumors, types 1 and 2 (46).
Conclusions
In our series patient age and gender (Caucasians) was similar to that described in the literature. The most common location was the stomach.
Overall incidence is probably increasing; regarding historical series, the percentage of gastroduodenal (many of them multifocal) and rectal CTs have increased likely due to the use of endoscopy, and because the initial historical series was predominantly surgical in nature (appendicular tumors predominated). The percentage of intestinal and appendicular carcinoids has decreased in contrast with literature reviews, but there is probably a bias since our series was based on endoscopy and echoendoscopy procedures. Most gastroduodenal and rectal CTs were treated endoscopically.
CS has decreased likely because of earlier CT diagnosis and treatment, and survival has slightly increased as a result of novel therapies.
References
1. MacDonald RA. A study of 356 carcinoids of the gastrointestinal tract: report of four new cases of the carcinoid síndrome. Am J Med 1956; 21: 867. [ Links ]
2. Sanders RJ, Axtell HK. Carcinoids of the gastrointestinal tract. Surg Ginecol and Obstetrics 1964; 119: 369-80. [ Links ]
3. Buchanan KD, Johnston CF, O'Hare M, Ardill JE, Shaw C, Collins JS, et al. Neuroendocrine tumors: a European view. Am J Med 1986; 81: 14-27. [ Links ]
4. Godwin J. Carcinoid tumors: an analysis of 2837 cases. Cancer 1975; 36: 560-9. [ Links ]
5. Modlin JM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997; 79: 813-29. [ Links ]
6. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13.715 carcinoid tumors. Cancer 2003; 97: 934-59. [ Links ]
7. Hemminke K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiological study from Sweden. Cancer 2001; 92: 2204-10. [ Links ]
8. Berge T, Linell F. Carcinoid tumours: frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand A 1976; 84: 322-30. [ Links ]
9. Lu L, Clemente C, Puig V, Mirada A. Tumor carcinoide. Análisis de 131 casos. Rev Clin Esp 1994; 194: 291-3. [ Links ]
10. Kang H, O'Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis 2007; 22: 183-9. [ Links ]
11. Younes RN. Neuroendocrine tumors: a registry of 1000 patients. Rev Assoc Med Bras 2008; 54: 305-7. [ Links ]
12. Kulke MH, Mayer RJ. Carcinoids tumors. New Engl J Med 1999; 340: 858-68. [ Links ]
13. Varas MJ. Tratamiento farmacológico de los apudomas gastroenteropancreáticos con el análogo de la somatostatina SMS 201-995 (octeotride). Rev Esp Enferm Dig 1991; 79: 95-8. [ Links ]
14. Varas MJ. Hiperplasia celular endocrina gástrica. BMJ (Ed. especial español) 1993; 5 (3): 55-7. [ Links ]
15. Gough DB, Thompson GB, Crotty TB, Donohue JH, Kvols LK, Carney JA, et al. Diverse clinical and pathologic features of gastric carcinoid and the relevance of hypergastrinemia. World J Surg 1994; 18: 473-80. [ Links ]
16. Thomas RM, Baybick JH, Elsayed Al M, Sobin LH. Gastric carcinoids. An immunohistochemical and clinicopathologic study of 104 patients. Cancer 1994; 73: 2053-8. [ Links ]
17. Modlin IM, Gilligan ChJ, Lawton GP, Tang LH, West AB, Darr U. Gastric carcinoids. The Yale experience. Arch Surg 1995; 130: 250-6. [ Links ]
18. Gilligan ChJ, Lawton GP, Tang LH, West AB, Modlin IM. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol 1995; 90: 338-52. [ Links ]
19. Modlin IM, Sandor A, Tang LH, Kidd M, Zelterman D. A 40-year analysis of 265 gastric carcinoids. Am J Gastroenterol 1997; 92: 633-8. [ Links ]
20. Yoshikane H, Goto H, Niwa Y, Matsui M, Ohashi S, Suzuki T, et al. Endoscopic resection of small duodenal carcinoid tumors with strip biopsy technique. Gastrointest Endosc 1998; 47: 466-70. [ Links ]
21. Mullen JT, Wang H, Yao JC, Lee JH, Perrier ND, Pisters PW, et al. Carcinoid tumors of duodenum. Surgery 2005; 138: 971-8. [ Links ]
22. Barclay THC, Schapira DV. Malignant tumors of the small intestine. Cancer 1983; 51: 878-81. [ Links ]
23. Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade análisis of a 3911 snall intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol 2007; 102: 1464-73. [ Links ]
24. Moertel CG, Weiland LH, Nagorney DM, Dockerty MB. Carcinoid tumor of the appendix: treatment and prognosis. New England J Med 1987; 317: 1699-701. [ Links ]
25. Roggo A, Wood WC, Ottinger LW. Carcinoid tumors of the appendix. Ann Surg 1993; 217: 385-90. [ Links ]
26. Varas MJ, García F, Barrios P, Borrell J. Tumores carcinoides del aparato digestivo. Med Clin 1985; 17: 715-6. [ Links ]
27. Martínez de Haro LF, Garca JA, Ortiz MA, et al. Tumores carcinoides digestivos. Presentación de 22 casos. Rev Esp Enferm Dig 1987; 72: 695-99. [ Links ]
28. Varas MJ. Tumores de los islotes pancreáticos. En: Vilardell F, Rodés J, Malagelada JR, Pajares JM, Pérez Mota A, editores. Enfermedades Digestivas 2. Madrid-Barcelona: Aula Médica; 1998. p. 1516-24. [ Links ]
29. Mani S, Modlin IM, Ballantyne G, Ahlman H. Carcinoids of the rectum. J Am College Surgeons 1994; 179: 231-48. [ Links ]
30. Soga J. Carcinoids and their variant endocrinomas. An analysis of 11842 reported cases. J Exp Clin Cancer Res 2003; 22: 517-30. [ Links ]
31. Teleky B. The prognosis of rectal carcinoid tumours. Int J Colorect Dis 1992; 7: 11-4. [ Links ]
32. Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, et al. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc 2003; 57: 583-7. [ Links ]
33. Martínez-Ares D, Souto-Ruzo J, Varas Lorenzo MJ, Espinós Pérez JC, Yáñez López J, Abad Belando R, et al. Endoscopic ultrasound-assisted endoscopic resection of carcinoid tumors of the gastrointestinal tract. Rev Esp Enferm Dig 2004; 96: 847-55. [ Links ]
34. Mashimo Y, Matsuda T, Uraoka T, Saito Y, Sano Y, Fu K, et al. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J Gastroenterol Hepatol 2008; 23: 218-21. [ Links ]
35. Martensson H, Nobin A, Sundler F. Carcinoid tumors in the gastrointestinal tract-an analysis of 156 cases. Acta Chir Scand 1983; 149: 607-16. [ Links ]
36. Modlin IM, Lye KD, Kidd M. A 50-year análisis of gastric carcinoids: small tumor or larger problem? Am J Gastroenterol 2003; 99: 23-32. [ Links ]
37. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer 2008; 113: 5-21. [ Links ]
38. Yao JC, Asan M, Phan A, Dahohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35.825 cases in the United States. J Clin Ocol 2008; 26: 3063-72. [ Links ]
39. Varas MJ. Neuroendocrine tumors -fascination and infrequency. Rev Esp Enferm Dig 2009; 101: 195-208. [ Links ]
40. Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumor. Ann Surg 2004; 240: 117-22. [ Links ]
41. Pinchot SN, Holen K, Sippel RS, Chen H, Carcinoids tumors. The Oncologist 2008; 13: 1255-69. [ Links ]
42. Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009; 41: 162-5. [ Links ]
43. Kaminski M, Polkowski M, Regula J. Prevalence and endoscopic features of rectal neuroendocrine tumors (carcinoids) among 50148 participants of the Polish colorectal-cancer screening programe. Gut 2007; 56 (Supl. III): A310. [ Links ]
44. Mulkeen A, Cha C. Gastric carcinoid. Curr Opin Oncol 2005; 17: 1-6. [ Links ]
45. Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC 2nd. A proposed staging system for gastric carcinoid tumors based on an analysis of 1543 patients. Ann Surg Oncol 2009; 16: 51-60. [ Links ]
46. Massironi S, Sciola V, Spampatti MP, Peracchi M, Conte D. Gastrics carcinoids: between underestimation and overtreatment. Word J Gastroenterol 2009; 15: 2177-83. [ Links ]
 Correspondence:
Correspondence:
M. J. Varas Lorenzo.
Centro Médico Teknon.
C/Marquesa de Vilallonga, 12. 08017 Barcelona. Spain.
e-mail: varas@dr.teknon.es
Received: 29-10-09.
Accepted: 25-02-10.











 texto en
texto en