Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.102 no.9 Madrid Set. 2010
Hepatitis B reactivation and current clinical impact
Reactivación de la hepatitis B y su impacto clínico actual
B. Álvarez Suárez1, J. de la Revilla Negro1, B. Ruiz Antorán2 and J. L. Calleja Panero1
1Servicio de Gastroenterología y Hepatología y 2Servicio de Farmacología Clínica. Hospital Universitario Puerta de Hierro. Majadahonda, Madrid. Universidad Autónoma de Madrid, Spain
ABSTRACT
Hepatitis B virus (HBV) reactivation results from increased viral replication in inactive carriers or patients with prior infection with HBV. Reactivation may occur spontaneously or secondary to immunomodulating or immunosuppressive chemotherapy. Reactivation may manifest with no symptoms but on occasion results in acute or even severe acute hepatitis. Prevention is the best management approach, hence HBV screening using serology should be performed for all patients undergoing any immunomodulating, immunosuppressive or chemotherapeutic treatment. Antiviral prophylaxis has proven effective in inactive carriers and in some patients with former infection with HBV undergoing selected immunosuppressive therapies.
Key words: Hepatitis B. Inmunosupresores. AntiTNF. Quimioterapia. Profilaxis.
RESUMEN
La reactivación del virus de la hepatitis B se debe a un aumento de la replicación del virus en pacientes portadores inactivos o con infecciones pasadas de VHB. La reactivación puede producirse espontáneamente o de manera secundaria a tratamientos de quimioterapia, inmunomoduladores o inmunosupresores. La reactivación puede manifestarse de manera asintomática pero en algunos casos puede causar hepatitis agudas e incluso hepatitis agudas graves. El mejor tratamiento es la prevención por lo que se debe realizar un cribado del VHB mediante una serología a todos los pacientes que vayan a someterse a cualquier tratamiento inmunomodulador, de quimioterapia o inmunosupresor. El tratamiento profiláctico antiviral ha demostrado ser eficaz en los pacientes portadores inactivos y en algunos pacientes con infecciones pasadas de VHB sometidos a ciertos tratamientos inmunosupresores.
Palabras clave: Hepatitis B. Inmunosupresores. AntiTNF. Quimioterapia. Profilaxis.
Introduction
The natural history of infection with HBV is highly heterogeneous and depends upon the interaction between viral, host, and environmental factors. When HBV infects a susceptible individual, it enters the hepatocyte and its DNA is incorporated into the cell's DNA, resulting in a covalent, circular, closed DNA form (DNAccc). DNAccc is transcribed in the hepatocyte's nucleus to form RNA, which later is translated in the cytoplasm to make up new viral particles. Thus, DNAccc acts as a template for the formation of new viruses that will infect other hepatocytes, and will remain in the nucleus until the liver cell is destroyed. B virus replication is not directly cytotoxic for cells. This accounts for the fact that despite high viral replication B virus carriers may be asymptomatic and display minimal liver damage.
During viral replication several antigens are expressed inside the liver cell; some are expressed in the cell membrane (HBcAg), some are released as circulating particles into the blood stream (HBsAg and HBeAg). The host's immune system recognizes these antigens as foreign, and triggers an immune response. On the one hand, a Th1 response is triggered that activates CD-8 lymphocytes and TNF-alpha formation, thus inducing the destruction of infected hepatocytes. On the other hand, a Th-2 response results where B-cells release antibodies against various antigens, and neutralize circulating viruses, thus preventing further liver cells from becoming infected.
If the immune response is effective all infected hepatocytes will be destroyed, and the infection will be resolved. If the immune response is inadequate, infection becomes chronic land progresses along 5 stages.
The immune tolerance stage is characterized by high viral replication (high B-virus DNA, positive HBsAg and HBeAg) resulting from poor immune response, which accounts for lack of histological damage and normal transaminases. The length of this stage depends on age at infection - it is long for perinatal infection and short for infection acquired during childhood or adulthood.
When immune tolerance subsides, the immune system destroys infected liver cells, which results in elevated transaminases, gradual viral load decrease, and liver necroinflammation. This is the immune activity stage, which clinically correlates with positive-HBeAg chronic hepatitis.
A relevant result of the immune activity stage is HBeAg seroconversion, characterized by HBeAg negativity, antiHBe positivity, low or undetectable HBV, and liver injury stabilization. This stage, known as the inactive carrier status, may last for years, and HBsAg seroconversion (past hepatitis) occurs in 1-2% of patients annually. However, these patients usually retain DNAccc in a number of hepatocytes (1).
Under certain circumstances, inactive carriers may undergo reactivation of viral replication while with positive antiHBe, which may be related to spontaneous or induced viral mutation, or loss of immune control. In this situation HBeAg remains negative, antiHBe is positive, viral DNA and transaminases increase, and there is greater liver cell damage. This is called HBeAg-negative chronic hepatitis.
In the last few years a new stage of chronic infection was defined, namely the negative HBsAg (or occult HBV) stage. It usually occurs in long-term inactive carriers whose immune system managed to clear HBsAg while circulating viral DNA persisted in very low levels or was exclusively detected in liver tissue (DNCccc). Such patients usually have no significant histological injury but may display sequels from prior necroinflammation. In selected immune suppression situations this group of patients, and exceptionally patients with past hepatitis B serology, may undergo HBV reactivation characterized by elevated viral load (≥ 1 log IU/ml), HBeAg and HBsAg recurrence, and increased transaminases above baseline values. Such reactivations are usually asymptomatic but may on occasion drift towards clinical hepatitis as defined by ALT 3 times its basal value together with the development of jaundice and other acute hepatitis symptoms (2,3).
Table I lists the serological profiles of chronic infection with HBV, and the scenarios where HBV reactivation may occur.
The changed relationship between the host's immune system and HBV is responsible for this reactivation. Up to 20-30% of inactive carriers may experience spontaneous reactivation related to the virus itself. Reactivation may also result from immune response changes induced by immunosuppression secondary to drug therapies, as is the case with solid organ and bone marrow transplants, chemotherapy for malignancies or the use of biological agents for immune-based conditions.
Epidemiological impact of chronic infection with HBV
It is estimated that 350-400 million people are HBsAg carriers worldwide. This population is distributed in three distinct endemicity areas based on HBsAg prevalence, with Southeast Asia and Africa exhibiting the highest prevalence. Spain is an intermediate endemicity country. In 1980, the prevalence of HBsAg and past hepatitis B carriers in Spain was 1.2-2% and 20%, respectively. Following the implementation of universal immunization programs against HBV, initially for adolescents then for newborns, the prevalence of HBsAg and past hepatitis B carriers in 2007 has decreased dramatically to 0.7% and 8.7%, respectively (4). These proportions are increased in advanced age groups and among the immigrant population. The prevalence of positive antiHBc in Spain among the population coming from high-endemicity areas such as Asia and Africa is 27.6% for Asians and 18.8% for Africans (5). Given the gradual increase of immigration rates in our country, this prevalence may be further increased. The prevalence of infection with occult B virus varies widely among studies: 0-89% in HIV patients and 0-36.8% in subjects undergoing hemodialysis (6).
As this epidemiological status represents a risk factor for HBV reactivation, the susceptible population is numerous, which prompts an optimization of prevention and management measures for this condition. On the other hand, improved prevention, health promotion, and therapies have managed to increase life expectancy for both the general population and HBsAg carriers, with a greater risk for developing malignancies in need of chemotherapy.
Epidemiological and clinical aspects of hepatitis B virus reactivation
Epidemiology and factors associated with reactivation
- Patients undergoing chemotherapy for solid and blood malignancies: hepatocarcinoma (7), nasopharyngeal tumors (8), breast cancer (9), leukemia (10), lymphoma (11), and bone marrow transplant (12,13).
- Pacients receiving immunomodulators (methotrexate) (14) or biologic agents (anti-TNF) (15,16);
- Patients undergoing immunosuppression for solid organ transplantation (17,18).
Given their immunosuppressive activity, any chemotherapy agent may favor reactivation (19). Table II lists chemotherapy drugs associated with B virus reactivation.
HBV reactivation in inactive carriers is 50% in patients undergoing chemotherapy (20-23), and 50-96% in patients undergoing renal or heart transplants (2,24); HBV reactivation in antiHBc-positive patients is lower, 6-10% and 0.9% in subjects receiving chemotherapy (25-26) and in patients with solid organ transplants, respectively (27).
Reactivation does not occur in all HBsAg-positive or antiHBc-positive carriers. Various factors related to the virus, host, and treatment type (immunosuppression extent) have been associated with B virus reactivation. Patients with a basal viral load higher than 104copies/ml and HBeAg carriers are more susceptible to reactivation (20,23-26,28), as well as males and younger individuals (20,28).
On the other hand, increased B virus reactivation has been described in patients undergoing aggressive immunosuppressive therapies such as chemotherapy combined with corticoids or the use of rituximab. In a study reported by Cheng et al (2003) patients with non-Hodgkin lymphoma who were positive for HBsAg were randomized to receive chemotherapy (epirubicin, cyclophosphamide, etoposide) with or without corticoids. This second group had a higher incidence of B virus reactivation (73% vs. 38%) and hepatitis (44% vs. 13%) (10).
Rituximab is a monoclonal antibody against antigen CD20, expressed on the surface of B-lymphocytes. This drug depletes B-cell population and antibody levels, and decreased the immune response, thus favoring B virus replication. This is why treatment with rituximab, whether associated with corticoids or otherwise, has a greater risk of reactivation, as shown in a study by Hui et al (2006) (25) where 12% of patients treated with rituximab had reactivation versus 1% among the rest of patients. Another, more recent study supports this; 25% of patients with lymphoma and positive AntiHBc receiving cyclophosphamide, doxorubicin, vincristin, prednisone (CHOP) and rituximab had their B virus reactivated as compared to no patients in the group treated exclusively with CHOP (26).
B virus reactivation in patients undergoing bone marrow transplantation is almost universal in HBsAg-positive patients, around 50% in antiHBc-positive patients, and 20% in patients with past hepatitis B (2,12,29-31). This correlates to high-dose chemotherapy administered during pretransplant conditioning and later consolidation.
Manifestations and diagnosis of B virus reactivation
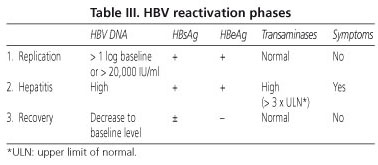
Reactivation may occur any time during therapy but is more common at therapy onset or after therapy completion because of immune reconstitution. Hepatitis virus B reactivation usually follows three stages (Table III):
1. Replication: increase in HBV DNA levels above 1 log from baseline or above 20,000 IU/ml, which corresponds to the transition from inactive carrier with undetectable viral load to active viral replication with HBeAg comeback and AntiHBe negativization (seroconversion phenomenon).
2. Hepatitis: increase in transaminase levels 3 times above baseline, which occurs at 2-3 weeks after DNA increase. In this stage, the patient may have symptoms such as asthenia, malaise, jaundice, and even severe acute hepatitis signs.
3. Recovery: in surviving patients B virus DNA and transaminases return to baseline usually following the discontinuation of cancer therapy or the introduction of antiviral therapy.
Reactivation in patients with positive antiHBc may manifest with only an increase in viral load with no elevated transaminases (2).
Clinical consequences of HBV reactivation
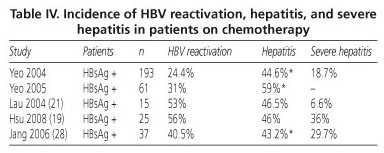
HBV reactivation may have two important results in patients on chemotherapy. On the one hand there is a risk for acute hepatitis, even severe acute hepatitis, with its associated morbidity and mortality. Direct mortality from B virus reactivation is primarily related to acute liver failure up to 1 year after treatment discontinuation. A meta-analysis by Kazt et al including 21 studies reports that 24-100% of patients with viral reactivation eventually develop acute hepatitis and 0-50% of mortality in these subjects was related to HBV. Table IV describes the incidence of reactivation, hepatitis, and severe hepatitis in major studies.
Another consequence of HBV reactivation is related with a need to delay or even discontinue chemotherapy, with the negative clinical impact it may have on total survival. In this same metaanalysis 10-19% of patients were withdrawn from treatment (32).
HBV reactivation in the setting of solid organ transplantation may also have serious effects, not only because of increased all-cause mortality (RR: 2.49; 95% CI: 1.64-3.78) but also because of increased risk for graft failure (RR: 1.44; 95% CI: 1.02-2.04), as noted in the metaanalysis by Fabrizi et al (33) in patients with renal transplant.
The clinical presentation of reactivation in patients undergoing bone marrow transplants usually manifests as serious hepatitis with delayed onset, after 1 to 3 years following transplantation (12-29-31).
In an attempt to prevent these unfavorable clinical outcomes current recommendations as per European guidelines and the latest consensus papers suggest serological HBV screening for all patients undergoing chemotherapy, immunosuppressive therapy (mainly transplanted individuals), and biological therapy (anti-tumor necrosis factor) (2, 34-35).
B virus screening in this population at risk is no universal practice, as demonstrated in the study by Tran et al. In a survey to 265 American oncologists, 20% never ordered HBV serology, and 30% only ordered this test for patients with abnormal liver function tests; in addition, for HBV carriers up to 15% of specialists prescribed no prophylactic therapy and made no referrals to hepatologists (36).
Estimated impact of HBV reactivation on populations at risk
To adequately approach the epidemiological and clinical significance of HBV reactivation in our country, we should accurately estimate the number of patients exposed to each drug class with potential for inducing reactivation. However, collecting such information is highly complex since no data are usually recorded on the use of such drugs by patients. Data are only available on the prevalence of related conditions (particularly on malignancies and transplants) and on the use of biological agents.
In an attempt to approach the relevant risk HBV reactivation may pose for public health, and to make the medical community aware of the need for HBV screening in populations at risk the following epidemiological data should be considered.
Patients exposed to antineoplastic drugs
The one approximation feasible to estimate the number of patients that may have received or be receiving any of the chemotherapy drugs listed in table II is based on the prevalence of neoplasms where these drugs are indicated. Given the high variability of drug classes in the list, these drugs are virtually indicated for all types of malignancy.
According to the report El Cáncer en España (37), published by SEOM (Sociedad Española de Oncología Médica) in January 2010, the current prevalence of cancer in Spain is currently estimated in over 1,500,000 people. The approximate percentage of patients receiving chemotherapy is around 25-30% (38). Hence, the total number of patients exposed to chemotherapy oscillates between 375,000 and 450,000.
In addition, data on the incidence of cancer are available from Spanish registries for the period 1998-2002 - between 324 and 511 cases/100,000 males/year and between 204 and 286 cases/100,000 females/year. Considering the percentage that will undergo treatment the incidence of Spanish men and women who will be exposed to chemotherapy may be estimated around 100-150 cases/100,000 males/year and 60-85 cases/100,000 females /year.
Exposure to biological therapies
An estimation of exposure to these drugs may be obtained from the number of containers used in the past year. This parameter is based on the number of DDDs (defined daily doses) per thousand population and day, which provides an estimation of the number of patients daily receiving a specific drug (39). DDD values are available from our Healthcare Area (Area 6, Madrid Community), which serves a population of around 621,395 inhabitants (http://vocaliamadrid.wordpress.com/area-6/). When all biologics available in Spain are considered, DDD/1000 population/day in 2009 is approximately 1; that is, in Area 6, in 2009, 1 out of 1000 inhabitants received daily a biologic agent DDD (Table V).
Another approach would consider prior information of patients visiting the outpatient medication dispensation unit, hospital pharmacology department, to collect their drug. In our Healthcare Area, during February 2010, 240 patients with a major indication for biological therapy (rheumatoid arthritis, spondilitis, psoriasis, Crohn's disease) collected some of these drugs at the hospital pharmacy. This number may increase by around 8-10 new patients per month, hence annual administration may be estimated in around 340 patients/year. Furthermore, in 2009, infliximab was dispensed to 160 people in-hospital (or at the day hospital). As our Area covers a population of 621,395 inhabitants, data may be extrapolated for an estimation of the overall population exposed to these drugs, resulting in 80 patients exposed per 100,000 population/year.
Exposure to immunosuppressive therapies among the transplanted population
All patients transplanted for any indication receive immunosuppressive therapy, which they will maintain for the rest of their lives.
To estimate exposure to immunosuppressants in this group of patients we shall use data relating to new exposures during the previous year exclusively, with respect to the total number of transplants performed in Spain for any indication (40).
Table VI shows data related to the number of solid organ transplants in the last three years.
As can be seen, around 4,000 new patients in the last year were exposed to immunosuppressants in Spain connected with solid organ transplants. We also know the number of patients with hematological conditions who underwent a bone marrow transplant in 2008 (2124 patients).
To conclude, based on all the above data, the real relevance of HBV reactivation among the Spanish susceptible population may be envisaged. In the transplant setting we have 4028 solid organ and 2012 bone marrow transplants (data from 2009 and 2008, respectively); regarding oncology around 46,000 patients/year will receive chemotherapy (approximately 100/100,000 inhabitants), whereas around 37,013 people will undergo treatment with biological therapies for primary indications (500/621,395 inhabitants). By adding all patient groups together, the overall Spanish population on drugs at risk of inducing HBV reactivation is 89,053 new subjects yearly. Of this population 623 will be HBV carriers (0.7% positive HBsAg in Spain) and 7748 will exhibit antiHBc positivity (8.7% of positive antiHBc in Spain). Should HBV screening recommendations fail to be implemented for these at-risk populations, and should preventive measures fail to be considered against reactivation, based on incidence numbers listed in table IV 187 new reactivation cases will occur yearly only among HBV carriers (positive HBsAg), 80 of which will clinically manifest as hepatitis with 5 to 25 having a serious outcome. When this analysis is performed on the antiHBc-positive population at risk numbers may rise 10-fold. (Spanish population in January 2010: 45,989,016 http://www.ine.es/jaxiBD/tabla.do)
Managing hepatitis B reactivation
Following a diagnosis with B virus reactivation chemotherapy should be first discontinued to allow immune system recovery. However, this intervention may lead to reduced treatment effectiveness and increased mortality. Therefore, all risks and benefits must be considered before making such a decision.
The next measure is antiviral therapy onset. Most reported studies used lamivudin because of its wide availability for years (41-46). However, lamivudin has a high rate of resistance (14% the first year, 65% at 5 years), which currently confines its use as a second-line therapy. First-choice drugs for the management of HBV reactivation should currently be those administered for chronic hepatitis B. Drugs with higher antiviral potency and lower long-term resistance rates should be administered initially. Two drugs currently meet these requirements and should be used for first-line therapy - entecavir and tenofovir. These drugs should be maintained indefinitely until virological and serological goals are reached as established by major consensus guidelines for the management of hepatitis B (34,47). Only two cases of HBV reactivation treated with first-line entecavir have been reported to date, with excellent clinical and virological results (48-49). A case of hepatitis B reactivation successfully treated with telbivudin was also recently reported (50).
Based on studies reporting the use of lamivudin, mortality rates associated with HBV despite lamivudin oscillate between 13% and 80%. Such high mortality highlights the relevance of reactivation preventing strategies as a measure to reduce complications and mortality in association with established reactivation.
Preventing B virus reactivation
Patients treated with chemotherapy agents (solid organ tumors and bone marrow transplants)
HBsAg-positive patients
Many retrospective and prospective studies with and without historical controls have confirmed a reduced incidence of B virus reactivation and associated mortality in inactive carriers prophylactically treated with nucleoside analogues, and lamivudin was the most widely used drug (19). Lamivudin inhibits viral respiration and decreases the risk of clinical hepatitis and its associated mortality. However only 3 prospective controlled studies have been reported in inactive carriers treated with chemotherapy for various indications.
The first prospective, randomized study was published in 2003 by Lau et al. Thirty patients with positive HBsAg and lymphoma were randomized to lamiudin 100 mg/day from one week prior to chemotherapy onset to 6 weeks later, or to receive said therapy in the presence of reactivation evidence. The various chemotherapy lines included cyclophosphamide, epirubicin, vincristin, prednisone (CEOP); adriamycin, bleomycin, vinblastin, dacarbazine (ABVD); cyclophosphamide, vincristin, procarbazine and prednisone (COPP) and CHOP. Fifty-three percent of patients not receiving preventive therapy developed B virus reactivation (7% hepatitis) versus no patient in the group with prophylaxis (22).
In another controlled study (20) 51 inactive carriers diagnosed with non-Hodgkin lymphoma were randomized to prophylaxis using lamivudin 100 mg/day since chemotherapy onset (CHOP) until two months after therapy completion, or to receive such therapy should hepatitis develop. In all, 7.7% of patients in the prophylaxis group and 48% of patients in the group with no prevention developed hepatitis because of viral reactivation. Upon lamivudin discontinuation, five patients developed viral reactivation, and two of them died from acute hepatitis.
The team of Jang et al randomized patients with HBV and hepatocarcinoma who would undergo chemoembolization to lamivudin 100mg/day from chemoembolization onset or no preventive therapy. Reactivation occurred in 2.8% of patients undergoing prophylaxis versus 29.7% of patients not randomized to lamivudin (23).
Scientific evidence favoring the use of lamivudin for preventing HBV reactivation was supported by two metaanalyses (32,51) including 35 studies. Both metaanalyses confirm a reduced hepatitis rates from viral reactivation, reduced all-cause mortality, and reduced B virus-related mortality in the prophylaxis group.
Therefore, all carriers to undergo chemotherapy should receive antiviral prophylaxis. No studies specifically designed to establish preventive therapy duration have been performed. An approximation may be derived from a review of two studies (52-53) including a total of 161 inactive HBV carriers with blood or solid malignancies who received prophylaxis with lamivudin from 1 week prior to chemotherapy onset to 1-6 months after chemotherapy completion. During follow-up without prophylaxis 13% to 24% of these patients had reactivation. On analyzing baseline factors associated with reactivation HBeAg-positive subjects with a high viral load (> 2,000-20,000 IU/ml) at baseline and treated for a blood malignancy were seen to have a higher risk for reactivation. Based on these observations, guidelines recommend that prophylaxis be initiated within 1 week prior to chemotherapy onset to 6-12 months after therapy completion.
Regarding the prophylactic antiviral drug of choice most studies have used lamivudin. However, reactivation events have been described in up to 9.6% of inactive carrier patients on prophylactic lamivudin, some of these related to demonstrated genotypal mutations against lamivudin. The risk for lamivudin failure has been associated with baseline viral load, and is significantly higher for viral loads above 20,000 IU/ml (HR 3.91 CI: 1.63-9.39, P = 0.002) (51). The development of new, more potent nucleoside analogues with reduced resistance will hopefully lead to their inclusion as measures for reactivation prevention.
Based on such evidence major hepatology societies (33,45,52) recommend that all HBsAg carriers bound to undergo chemotherapy should receive prophylaxis with lamivudin when chemotherapy has an estimated duration of 12 months and baseline viral load is undetectable. In contrast, should therapy duration be shorter than 12 months, entecavir or tenofovir are recommended. During prophylaxis ALT levels should be monitored every 4 weeks, and HBV DNA every 3 months or in case of elevated ALT. Should viral load increase 1 log above baseline levels a potential development of resistance should be considered, and antiviral therapy should be modified according to consensus guidelines. Given the risk for late reactivation following prophylaxis discontinuation laboratory surveillance should be ongoing to 1 year after therapy completion.
Patients with HBcAc +/- HBsAc
Four reported studies assessed the outcome of patients with positive AntiHBc +/- AntiHBs serology on chemotherapy. In the one prospective, observational study (Borentain) 8% of patients had reactivation (7/84), three of which (47%) died from liver failure despite therapy with lamivudin (55). The other studies (25-26,56) included a total of 303 patients diagnosed with blood malignancies. Reactivation percentages oscillated between 3% and 24%, and the only common factor for this phenomenon was rituximab (25-26).
Other factors related to reactivation included the presence of detectable HBV DNA pretreatment (25), receiving more than just one chemotherapy line, having had a BMT, or AntiHBs titers below 10 IU/ml in patients with past hepatitis B serology (55).
As previously discussed, patients undergoing bone marrow transplants have a higher reactivation rate due to aggressive immunosuppressive therapy, and up to 50% of AntiHBc-positive patients may develop viral reactivation (2).
Based on this information, prophylaxis recommendations for HBsAg-negative/AntiHBc- positive patients bound to receive chemotherapy and/or immunosuppression relies on ALT and viral load monitorization during therapy, with nucleoside analogue initiation should viral load increase. Patients who will undergo a bone marrow transplant or aggressive immunosuppression should receive prophylaxis with lamivudin from the start (2, 34).
Patients on biologic therapies
Inactive carriers or subjects with positive antiHBc and rheumatological, skin, gastrointestinal or allergic conditions on immunosuppressants may develop HBV reactivation but with a lower incidence (2). Reactivation associated with immunomodulators is rare but has been described (14,57-58). However, the drug class most commonly associated with reactivation is that of anti-TNF alpha antibodies. Killer T-cells together with TNF-alpha and interferon gamma play a key role in the inhibition of HBV replication and clearance of infected liver cells. Interaction between TNF-alpha and IFN-gamma suppresses viral replication and helps eliminate HBV by acting on all its antigens. TNF-alpha destroys infected liver cells by activating apoptotic mechanisms. These antiviral effects of TNF-alpha may be blocked using monoclonal anti-TNF-alpha antibodies, which reactivate viral replication and the risk for clinical hepatitis (1,15). Anti-TNF-alpha drugs available in Spain include infliximab, adalimumab, and etanercept.
The incidence of reactivation is uncertain since no prospective studies exist, and most reports refer to case series. Experience with infliximab is widest, and includes 21 patients (14,58-68). No patient receiving prophylaxis with lamivudin or entecavir (60) had a reactivation event, whereas most untreated patients had a clinical reactivation event, some with fulminant hepatitis, death, or liver transplant (61).
Evidence available with etanercept is smaller. From 2006 to 2009 7 HBV carrying patients have been reported with rheumatological conditions on etanercept. Only one severe reactivation event was described in a patient with past hepatitis B serology, whose response to lamivudin was excellent (68). The remaining patients (15, 68, 70) had exclusively virological reactivations with no clinical impact and a good outcome. No patient on prophylactic lamivudin had reactivation (68).
Regarding adalimumab only two reports exist including 3 patients with HBV (70,71). Only one had reactivation without clinical hepatitis, one received prophylaxis and had no reactivation, and one has no reactivation data after follow-up.
Two studies have been reported in an attempt to assess the true clinical impact HBV reactivation may have on the overall population on anti-TNF drugs. One, which was performed in 103 patients with rheumatological disease on one of the 3 mentioned anti-TNFs, reported one reactivation event among all 8 inactive carriers in the cohort (72). The patient had received infliximab. None of the remaining patients treated with adalimumab or etanercept experienced reactivation. The second study, performed in Spain on a series of 80 patients with inflammatory bowel disease on infliximab, detected 3 HBsAg carriers. The two patients on infliximab who received no prophylaxis against HBV experienced viral reactivation whereas the patient who received lamivudin had no reactivation (16).
To conclude, it may be stated that the indication of prophylaxis for patients on classic immunosuppressants (steroids, azathioprine or methotrexate) is not clearly established given the low rate of reactivation. The use of prophylaxis in inactive carriers to be treated with infliximab is indicated because of available evidence. While experience with adalimumab and etanercept does not seem to demonstrate such a high risk for reactivation, the paucity of reported cases and described mechanisms of action in this drug class renders prophylaxis desirable. The antiviral drug of choice for prophylaxis in this group of patients is not clearly established. Since anti-TNF therapy is usually prolonged, and given the known resistance rate for lamivudin, it seems only logical that drugs with a low resistance profile in the mid-long term should be used, including entecavir and tenofovir.
Patients on immunosuppressants for solid organ transplant
This section will only discuss the available experience with non-liver solid organ transplants. The prophylaxis and therapy of liver transplant for HBV involves such complex features that it deserves a specific chapter.
Patients undergoing solid organ transplants usually require prolonged immunosuppression to prevent rejection, which may favor HBV reactivation.
Because of this, transaminases, HBV serology, and HBV viral load in the case of HBV carriers should all be measured in patients assessed for inclusion in wait lists for renal, heart or lung transplantation. For HBsAg-positive patients, therapy should start with oral antiviral drugs, at transplantation when organs come from cadaveric donors or 4-6 weeks before transplant in the case of living donors. ALT and HBV DNA must be monitored every 3 months after transplant, and more closely during immunosuppression changes or augmentation. While studies have been performed with lamivudin, its high rate of resistance and the prolonged duration of immunosuppressive therapy advise that antivirals with a lower resistance rate (entecavir or tenofovir) be recommended at present (34-35). Patients with past hepatitis B serology (positive AntiHBs and AntiHBc) should have their antiHBs titer measured. For titers higher than 100 IU/ml follow-up with transaminase monitoring is recommended; for titers lower than 100 IU/ml a new immunization should be considered. In patients positive for AntiHBc and negative for AntiHBs the potential for clinical reactivation is smaller. In a study in 38 antiHBc-positive/HBsAg-negative patients undergoing solid organ transplantation, 44% had a detectable viral load post-transplant but only 5% became positive for HBsAg and none had clinical evidence of hepatitis (73). Based on these results, prophylaxis should only be considered for maximum immunosuppression periods, including the management of rejection episodes (35).
Conclusions
The risk for reactivation in inactive carriers or antiHBc-positive patients undergoing immunosuppressive therapy is high. This may result in increased patient morbidity and mortality. Thus, all patients scheduled to undergo immunosuppressive therapy or transplant should be tested for HBV serology.
A patient with negative serology is susceptible to infection and must be immunized.
A patient positive for HBsAg should have HBV DNA measured. When HBV DNA is higher than 2000 IU/ml conventional antiviral therapy should be administered with first-choice nucleoside analogues. When HBV DNA is lower than 2000 IU/ml or undetectable prophylaxis with lamivudin is recommended when estimated therapy duration is shorter than 12 months, or with entecavir/tenofovir is therapy will last longer than 12 months.
If a patient is positive for AntiHBc with or without AntiHBs HBV DNA should be tested. If DNA is detectable or immunosuppressive therapy is highly aggressive (bone marrow transplant) prophylaxis is recommended. Otherwise transaminases and HBV DNA should be monitored every three months for the duration of immunosuppressive therapy, with antivirals being initiated should viral load increase.
Figure 1 shows the algorithm recommended for the management of patients undergoing immunosuppressive therapy.
References
1. Ganem D, Prince AM. Hepatitis B Virus Infection - Natural History an Clinical Consequences. New England Journal of Medicine 2004: 350-1118-29. [ Links ]
2. Hoofnagle J. Reactivation of Hepatitis B. Hepatology 2009; 49 (5 Suppl): S156-65. [ Links ]
3. Simón Marco MA. Historia natural. Consenso para el tratamiento del virus B y C. Gastroenterología y Hepatología 2006; 29(Supl 2): 7-10. [ Links ]
4. Salleras L, Domínguez A, Bruguera M, Plans P, Costa J, Cardeñosa N, et al. Declining prevalence of hepatitis B virus infection in Catalonia (Spain) 12 years after the introduction of universal vaccination. Vaccine 2007; 25: 8726. [ Links ]
5. Salleras L, Dominguez A, Bruguera M, Plans P, Espuñes J, Costa J, et al. Seroepidemiology of hepatitis B virus infection in pregnant women in Catalonia (Spain). J Clin Virol 2009; 44: 329-32. [ Links ]
6. Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol 2007; 46: 160-70. [ Links ]
7. Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systematic chematherapy. Ann Oncol 2004; 15: 1661-6. [ Links ]
8. Yeo W, Hui EP, Chan AT, Ho WM, Lam KC, Chan PK, et al. Prevention of hepatitis B virus reactivation in patients with nasopharyngeal carcinoma with lamivudine. Am J Clin Oncol 2005; 379-84. [ Links ]
9. Dai MS, Wu PF, Shyu RY, Lu JJ, Chao TY. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int 2004; 24: 540-6. [ Links ]
10. Cheng A, Hsiung C, Su I, Chen P, Chang M, Tsao C, et al. Steroid-Free Chemotherapy Decreases Risk of Hepatitis B virus (HBV) Reactivation in HBV-Carriers with lymphoma. Hepatology 2003; 37: 1320-8. [ Links ]
11. Persico M, De Marino F, Russo GD, Morante A, Rotoli B, Torella R, et al. Efficacy of lamivudine to prevent hepatitis reactivation in hepatitis B virus-infected patients treated for non-Hodgkin lymphoma. Blood 2002; 15: 724-5. [ Links ]
12. Onozawa M, Hashino S, Izumiyama K, Kahata K, Chuma M, Mori A, et al. Progresive disappearance of anti-hepatitis Surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation 2005; 79: 616-9. [ Links ]
13. Otero López-Cubero S, Espigado I, Aguilar Reina J, Parody R. Reactivation of HBV following allogeneic bone marrow trasplantation: new Outlook (the hepatitis B virus and the bone marrow trasplant). Rev Esp Enferm Dig 1999; 91: 229-230. [ Links ]
14. Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 2003; 62: 686-7. [ Links ]
15. Carroll MB, Bond MI. Use of Tumor Necrosis Factor α Inhibitors in Patients with Chronic Hepatitis B Infection. Semin Arthritis Rheum 2007; 38: 208-17. [ Links ]
16. Esteve M, Saro C, Gonzalez-Huix F, Suárez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut 2004; 53: 1363-5. [ Links ]
17. Marcellin P, Giostra E, Martino-Peignoux M, Loriot MA, Jaengle ML, Wolf P. Redevelopment of hepatitis B surface antigen after renal trasplantation. Gastroenterology 1991; 100: 1432-4 [ Links ]
18. Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, et al. NIDDK Liver Transplantation Datebase. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. Gastroenterology 1997; 113: 1668-74 [ Links ]
19. Yeo W, Johnson PJ. Diagnosis, Prevention and Management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006; 43: 209-20. [ Links ]
20. Hsu C, Hsiung CA, Su IJ, Wang WS, Lin SF, Lin TH, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology 2008; 47: 844-53. [ Links ]
21. Lok AS, Liang RH, Chiu EK. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy: report of a prospective study. Gastroenterology 1991; 100: 182-8. [ Links ]
22. Lau G, Yiu H, Fong D, Cheng H, Au W, Lai L, et al. Early is Superior to Deferred Preemptive Lamivudine Therapy for Hepatitis B Patients Undergoing Chemotherapy. Gastroenterology 2003; 125: 1742-9. [ Links ]
23. Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randominzed controlled study of preemptive lamivudine in patients receiving Transarterial Chemo-Lipiodilization. Hepatology 2006; 43: 233-40. [ Links ]
24. Ko WJ, Chou NK, Hsu RB, Chen YS, Wang SS, Chu SH, et al. Hepatitis B Virus infection in heart transplant recipients in hepatitis B endemic area. J Heart Lung Trasplant 2001; 20: 865-75. [ Links ]
25. Hui CK, Cheung WW, Zhang H, Au W, Yueng Y, Leung A, et al. Kinetics and Risk of De Novo Hepatitis B infection in HBsAg-negative Patients Undergoing Cytotoxic Chemotherapy. Gastroenterology 2006; 131: 59-68. [ Links ]
26. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009; 27: 605-11. [ Links ]
27. Berger A, Preiser W, Kachel HG, Sturmer M, Doerr H. HBV reactivation after kidney transplantation. J Clin Virol 2005; 32: 162-5. [ Links ]
28. Yeo W, Chan P, Zhong S, Ho W, Steinberg JL, Tam L, et al. Frequency of Hepatitis B Virus Reactivation in Cancer Patients Undergoing Cytotoxic Chemotherapy: A Prospective Study of 626 patients with Identification of Risk Factors. J Med Virol 2000; 62: 299-307. [ Links ]
29. Dhedin N, Douvin C, Kuentz M, Saint Marc MF, Remen O, Rieux C, et al. Reverse seroconversion of hepatitis B alter allogeneic bone marrow transplantation: a retrospective study of 37 patients with pretransplant antiHBs and antiHBc. Transplantation 1998; 66: 616-9. [ Links ]
30. Seth P, Alrajhi AA, Kagevi I, Chaudhary MA, Colcol E, Sahovic E, et al. Hepatitis B reactivation with clinical flare in allogeneic stem cell transplant with chronic graft-versus-host disease. Bone Marrow Transplantion 2002; 30: 189-94. [ Links ]
31. A. Knöll, S. Boehm, J. Hahn, E. Holler, W. Jilg. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2004; 33: 925-9. [ Links ]
32. Katz L, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R. Lamivudine prevents reactivation of hepatitis B and reduce mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepatitis 2008; 15: 89-102. [ Links ]
33. Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G. HBsAg seropositive status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant 2005; 5: 2913-21. [ Links ]
34. European Association of the study of the liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. J Hepatol 2009; 50: 227-42. [ Links ]
35. Barclay S, Pol S, Multimer D, Benhamou Y, Mills P, Hayes P, et al. Erratum to "The management of chronic hepatitis B in the immunocompromised patient: Recommendations from a single topic meeting". Journal of Clinical Virology 2008; 42: 104-15. [ Links ]
36. Tran TT, Rakoski MO, Martin P, Poordad F. Screening for hepatitis B in chemotherapy patients: Surrey of current oncology practices. Aliment Pharmacol Ther 2010; 31: 240-6. [ Links ]
37. El Cáncer en España. SEOM. Enero 2010. http://www.seom.org/es/prensa/el-cancer-en-espanyacom. Acceso el 15 de marzo de 2010. [ Links ]
38. Cabanes Doménech A, Pérez-Gómez B, Aragonés N, Pollán N, López-Abente G. La situación del cáncer en España. 1975-2006. ISCIII. Madrid 2009. [ Links ]
39. WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment, 2010. Oslo, 2009. [ Links ]
40. Evolución de la Actividad de Donación y Trasplante en España. Organización Nacional de Trasplantes. http://www.ont.es/Documents/Datos donacion y trasplante 2009 revisada.pdf Acceso 15 de marzo 2010. [ Links ]
41. Yeo W, Steinberg JL, Tam JS, Chan PK, Leung NW, Lam KC, et al. Lamivudine in treatment of hepatitis B reactivation during cytotoxic chemoterapy. J Med Virol 1999; 59: 263-9. [ Links ]
42. Petrelli E, Balducci M, Pieretti C, Rocchi MB, Clementi M, Manzin A. Lamivudine treatment failure in preventing fatal outcome of the novo severe acute hepatitis B in patients with haematological diseases. J Hepatol 2001; 35: 823-6. [ Links ]
43. Liao CA, Lee CH, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin's lymphoma. Br J Haematol 2002; 116: 166-9. [ Links ]
44. Marín E, Rendon P, De Diego L, Soria MJ, Martínez MC, Martín L. Role of lamivudine in reactivation of hepatitis B virus infection in immunodepressed patients. Rev Esp Enferm Dig 2003; 95: 799-803. [ Links ]
45. Llop E, de la Revilla J, Pons F, Peñas B, Martínez JL, Abreu L et al. Decrease in viral load at weeks 12 and 24 in patients in chronic hepatitis B treated with lamivudine o adefovir predicts virological response at week 48. Rev Esp Enferm Dig 2009; 101: 763-7. [ Links ]
46. Simpon ND, Simpon PW, Ahmed AM, Nguyen MH, Garcia G, Keeffe EB, et al. Prophylaxis against chemotherapy-induced reactivation of hepatitis B virus infecction with lamivudine. J Clin Gastroenterol 2003; 37: 68-71. [ Links ]
47. Lok A, McMahon B. Chronic Hepatitis B: Uptodate 2009. Hepatology 2009; 50: 1-36. [ Links ]
48. Sánchez MJ, Buti M, Homs M, Palacios A, Rodríguez-Frías F, Esteban R. Successful use of entecavir for a severe a case of reactivation of hepatitis B virus following polychemotherapy containing rituximab. J Hepatol 2009; 51: 1091-6. [ Links ]
49. Colson P, Borentain P, Cose D, Chebannon C, Tamalet C, Gerolami R. Entecavir as a first line treatment for HBV reactivation following polychemotherapy for lymphoma. Br J Hematol 2008; 143: 148-50. [ Links ]
50. Montineri A, Nigro L, Chiarenza A, Larocca L, La Rosa R, Lacobello C, et al. Telbivudine use in a patient affected by occult hepatitis B virus and B-cell non-Hodgkin lymphoma. Leuk Lymphoma 2010; 51: 554-7. [ Links ]
51. Loomba R, Rowley A, Wesley R, Liang J, Hoofnagle J, Pucino F, et al. Systematic Review: The effect of Preventive Lamivudine on Hepatitis B Reactivation during Chemotherapy. Ann Intern Med 2008; 148: 519-28. [ Links ]
52. Hui CK, Cheung WW, Lie AK, Zhang HY, Yueng YH, Wong BC, et al. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut 2006; 55: 1208-9 [ Links ]
53. Kim IK, Kim W, Kim BS, Jung YJ, Beng Jeong JB, Kim BG, et al. Clinical prediction of failure in antiviral prophylaxis for patients with hepatitis B infection undergoing cytotoxic chemotherapy for malignant tumors. J Hepatol 2009 (Supl. 1): S332. [ Links ]
54. M. Torres Salinas. Hepatitis B en situaciones especiales. Consenso para el tratamiento de virus B y B. Gastroenterología y Hepatología 2006; 29(Supl. 2): 76-81. [ Links ]
55. Borentain P, Colsen P, Cose D, Bories E, Charbonnier A, Stoppa AM, et al. Clinical and virological factors associated with hepatitis B virus reactivation in HBsAg negative and anti-HBc antibodies-positive patients undergoing chemotherapy and/or autologous stem transplantation for cancer. J Viral Hepat. Epud ahead of print. [ Links ]
56. Francisci D, Falcinelli F, Schianroli E, Capponi M, Belflari B, Flenghi L, et al. Management of hepatitis B virus reactivation in patients with haematological malignancies treated with chemotherapy. Infection 2010; 38: 58-61 [ Links ]
57. Ito S, Nakazono K, Murasawa A, Mita Y, Hata K, Saito N et al. Development of fulminant hepatitis B (precore variante mutante type) after the discontinuation of low-dose methotrexate therapy in a rheumatoid arthritis patients. Arthritis Rheum 2001; 44: 339-42. [ Links ]
58. Calabrese LH, Zein NN, Vassilopoulos D. Hepatitis B virus (HBV) reactivation with immunosuppressive therapy in rheumatic disease: assessment and preventive strategies. Ann Rheum Dis. 2006; 65: 983-9. [ Links ]
59. Anelli MG, Torres DD, Manno C, Scioscie C, Ionnane F, Covelli F, et al. Improvement of renal function and disappearance of hepatitis B virus ADN in a patients with rheumatoid arthritis and renal amyloidosis following treatment with infliximab. Arthitis Rheuma 2005; 52: 2519-20. [ Links ]
60. Conde-Taboada A, Pedraz J, Campos L, López-Bran E. Infliximab treatment for severe psoriasis in a patient with active hepatitis B virus infection. J Am Acad Dermatol 2009; 60: 1077-9. [ Links ]
61. Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant Hepatitis After Infliximab in a Patient with Hepatitis B Virus Treated for an Adult Onset Still's disease. J Rheumatol 2003; 30: 1624-5. [ Links ]
62. Ojiro K, Naganuma M, Ebinuma H, Kunimoto H, Tada S, Ogata H, et al. Reactivation of hepatitis B in a patient with Crohn's disease treated using Infliximab. J Gastroenterol 2008; 43: 397-401. [ Links ]
63. Millonig G, Kern M, Ludwiczek O, Nachbaur K, Vogel W. Subfulminant hepatitis B after infliximab in Crohn's disease: Need for HBV-screening? World J Gastroenterol 2006; 12: 974-6. [ Links ]
64. Oniankitan O, Duvoux C, Challine D, Mallat A, Chevalier X, et al. Infliximab Therapy for Rheumatic Diseases in Patients with Chronic Hepatitis B or C chronic HBV or HCV infection. J Rheumatol 2004;31:107-9. [ Links ]
65. Roux CH, Brocq O, Breuil V, Albert C, Euller-Ziegler L. Safety of anti-TNF-a therapy in rheumatoid arthritis and spondylarthropathies with concurrent B or C chronic hepatitis. Rheumatol 2006; 45: 1294-7. [ Links ]
66. Sakellariou TG, Chatzigiannis I. Long-term anti-TNFα therapy for ankylosing spondylitis in two patients with chronic HBV infection. Clin Rheumatol 2007; 26: 950-2. [ Links ]
67. Ueno Y, Tanaka S, Shimamoto M, Miyanaka Y, Hiyana T, Ito M, et al. Infliximab Therapy for Crohn's Disease in a Patient with Chronic Hepatitis B. Dig Dis Sci 2005; 50: 163-6. [ Links ]
68. Wendling D, Di Martino V, Prati C, Toussirot E, Herbein G. Spondyloarthropathy and chronic B hepatitis. Effect of anti-TNF therapy. Joint Bone Spine 2009; 76: 308-11. [ Links ]
69. Martínez Montiel P, Solis JA, Chirinos JA, Casis B, Sánchez F, Rodríguez S. Hepatitis B virus reactivation during therapy with etanercept in an HBsAg-negative and anti-HBs-positive patient. Liver International 2008; 28: 718-20. [ Links ]
70. Li S, Kaur PP, Chan V, Berney S. Use of tumor necrosis factor-α (TNF-α) antagonists infliximab, etanercept, and adalimumab in patients with concurrent rheumatoid arthritis and hepatitis B or hepatitis C: a retrospective record review of 11 patients. Clin Rheumatol 2009; 28: 787-91. [ Links ]
71. Kaur PP, Chan VC, Berney SN. Histological evaluation of liver in two rheumatoid arthritis patients with chronic hepatitis B and C treated with TNF-alpha blockade: case reports. Clin Rheumatol 2008; 27: 1069-71. [ Links ]
72. Kim SJ, Park MC, Park YB, Lee SK. Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol. 2009; 36: 2416-20. [ Links ]
73. Knöll A, Pietrzyk M, Loss M, Goetz WA, Jilg W. Solid-organ transplantation in HBsAg-negative patients with antibodies to HBV core antigen: low risk of HBV reactivation. Transplantation 2005; 79: 1631-3. [ Links ]
 Correspondence:
Correspondence:
José Luis Calleja Panero.
Servicio de Aparato Digestivo.
Hospital Universitario Puerta de Hierro.
C/ Manuel de Falla, 1.
28222 Majadahonda. Madrid, Spain.
e-mail: jlcpan@terra.es
Received: 17-06-10.
Accepted: 17-06-10.











 texto em
texto em