Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.103 no.2 Madrid Fev. 2011
PillCam© Colon Capsule for the study of colonic pathology in clinical practice. Study of agreement with colonoscopy
Capsula colónica PillCam© para el estudio de la patología del colon en la práctica clínica
J. M. Herrerías-Gutiérrez, F. Argüelles-Arias, A. Caunedo-Álvarez, M. San-Juan-Acosta, J. Romero-Vázquez, J. M. García-Montes and F. Pellicer-Bautista
Digestive Department. Virgen Macarena University Hospital. Seville, Spain
ABSTRACT
Introduction: several studies have pointed out the effectiveness of the PillCam© colon capsule endoscopy (CCE) compared with the colonoscopy in the study of the colonic pathology.
Aims and methods: the objective of our study was to assess the agreement in the diagnosis of CCE with conventional colonoscopy as well as its sensitivity and specificity, and to describe the findings of the CCE in our clinical practice. Consecutive patients with abdominal symptoms were included in the study. The CCE was performed as previously reported (with PEG and sodium phosphate as laxative agents). The nature and location of the findings, colonic transit time, complications, cleanliness degree and consistency with diagnostic colonoscopy, when performed, were analyzed.
Results: a total of 144 subjects (67 women and 77 men); (52.17±16.71 years) with the following indications were included: screening of Colorectal cancer (88 patients), control after polipectomy (24), incomplete colonoscopy (7), rectal bleeding (10), anemia (8), diarrhea (7). The CCE exploration was complete in 134/144 cases (93%), with no case of retention. The preparation was good-very good in 88/134 (65,6%), fair in 26/134 (19,4%) and poor in 20/134 (15%) of the cases. The average colonic transit was of 140.76 min (9-603). Any adverse effect was notified.
In 44 cases a colonoscopy was carried out after CCE (results were hidden from another endoscopist). Compared to colonoscopy, the rate of agreement was 75,6%, the sensitivity was 84% and the specificity 62,5%, PPV was 77,7% and NPV was 71,4 %.
The colonic findings in 134 CCE were: in 34 cases CCE it did not show lesions, diverticulosis in 63 explorations, polyps in 43, angiodysplasias in 15, Crohn's Disease in 9 and ulcerative colitis in other 8 cases.
Conclusions: the CCE is an effective and reliable technique for the detection of lesions in colon, and because of its high agree-ment with the colonoscopy, it could be useful in clinical practice. Further studies with large seria and cost-effectiveness analysis are needed to confirm these data.
Introduction
Capsule endoscopy has been established as the diagnosis of obscure gastrointestinal bleeding and in other small bowel suspected lesions (1-3) and it is known to be useful only for the study of small bowel mucosa injuried (4). Following the path marked by this capsule, a colon capsule endoscopy has been developed. Several studies have pointed out the effectiveness of the PillCam© colon capsule endoscopy (CCE) compared to the colonoscopy in the study of the colonic pathology (5,6).
The CCE is similar to the conventional capsule but has two cameras which are able to record video images from both ends. The device measures 31 by 11 mms and acquires images at a rate of 4 frames per second. The pre-programmed "sleep" mode allows recording of images from the esophagus and the stomach for 3 minutes and after that capsule switches to sleep mode for 1 hour 45 minutes, so that it saves battery. During this period, the capsule is likely to transit most of the small bowel and reaches approximately the level of terminal ileum. Recording and downloading of data are similar to those for small-bowel capsule endoscopy (7).
The high-priority objective of CCE is the "screening" of colorectal cancer in the risk population and appears to be a promising new modality for colonic evaluation. In order to find out the sensitivity and specificity of the CCE compared to colonoscopy in screening colorectal cancer some studies have been developed (5,6,8,9).
In addition, CCE may be a new technique to evaluate colonic lesions not only adenomas. It could be a good alternative in patients refusing conventional colonoscopy or when it is contraindicated. This is the first study that compares other lesions not only polyps to colonoscopy and moreover we describe our findings in all the procedures we have carried out with the new CCE.
Aims
Based on the aboved mentioned studies, it seems that CCE could be a new non-invasive modality to visualize the colon. Therefore, the main objective of our study was to evaluate the detection rates and the agreement of colonic pathological conditions, not only polyps, using CCE compared to conventional colonoscopy as well as its sensitivity and specificity.
The secondary objective was to describe the findings of CCE in our clinical practice, efficacy of colon cleansing preparation and adverse events detected with the CCE.
Material and methods
Study design
This was a prospective study divided in two parts:
- The first part was a prospective study in which we compare CCE with colonoscopy for the detection of colonic disease.
- And the second part was a prospective and only descriptive study in which we describe our CCE fin-dings in patients with suspicious of colonic lesions.
The inclusion criteria were people between 18 and 80 years old who had been referred for colorectal cancer screening, control after polipectomy or suffered digestive symptoms (rectal bleeding, anemia or diarrhea). The majority of these patients did not want to undergo a conventional colonoscopy.
Exclusion criteria were similar to those used in conventional capsule endoscopy: dysphagia, suspected or known small bowel or colonic strictures, pregnancy, abdominal surgery in the past 6 months, a life threatening condition, allergic to one of the laxative included in the preparation or inability to provide informed consent.
All subjects included in this study signed the written informed consent prior to study enrolment.
The Pillcam® Colon Capsule
The Pillcam® Colon Capsule (Given Imaging) used in this study is the conventional capsule used in the previous studies that evaluated this new device. It has two cameras and a ten-hour life battery. After three minutes running, the capsule turns off for a period of two hours and then wakes up and restarts the transmission of images.
Colon preparation and assessment of bowel cleanliness
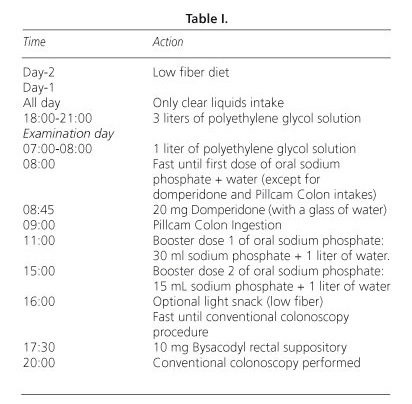
The CCE was performed as previously reported (with PEG and sodium phosphate as laxative agents) (5,6). Patients were asked to maintain a low fiber diet 2 days before capsule ingestion. Once the capsule was swallowed, additional laxative and prokinetic agents were provided to the patients (Table I).
The nature and location of the findings, colonic transit time, complications, degree of cleanliness and consistency with diagnostic colonoscopy, when it was performed, were analyzed.
The grading scale used to assess colon cleanliness at CCE was the same as used in previous studies (5,6) (Table II).
Comparison of capsule endoscopy and conventional colonoscopy
Conventional colonoscopy was performed after the capsule endoscopy, on the same or following day (following CCE excretion). The physician performing the capsule procedure and reading the capsule and the physician performing the conventional colonoscopy study were blinded for the other technique. When a polyp was seen with the colonoscopy, it was removed and recorded by location and by size.
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), of the Pillcam Co-lon capsule endoscopy versus conventional colonoscopy were calculated.
A second descriptive analysis was done in the non-comparative study.
Results
A total of 144 subjects were included in the study (67 women and 77 men); (52.17±16.71 years). The indications were: screening of colorectal cancer (88 patients), control after polipectomy (24), incomplete colonoscopy (7), rectal bleeding (10), anemia (8), diarrhea (7). In 44 ca-ses a colonoscopy was carried out after CCE (results hidden from another endoscopist). In 3 cases the comparison was not possible (in 2 cases because the capsule examination was not completed and in 1 case because colonoscopy was not concluded). The rest of patients (100 patients) refused conventional colonoscopy so the possibility to undergo a CEE was offered to them.
Compared to colonoscopy, the rate of agreement was 75,6%, the sensitivity was 84% and the specificity 62,5%, PPV was 77,7% and NPV was 71,4 % (Table III).
In 4 cases CCE was positive with negative colonoscopy. Two cases were diverticulosis and 2 had one angiodysplasia that was not seen by colonoscopy. Concerning detection of polyps, CCE detected 19 polyps (two of them not detected by colonoscopy) and colonoscopy detected 19 polyps (two of them not detected by CCE).
The exploration with CCE was completed in 134/144 cases (93%), without any case of retention. The preparation was good-very good in 88/134 (65,6%), fair in 26/134 (19,4%) and poor in 20/134 (15%) of the cases. The average colonic transit was 140.76 min (9-603). Any adverse effect was notified.
The colonic findings in 134 CCE were: in 34 cases CCE did not show lesions, diverticulosis in 63 explorations, polyps in 43, angiodysplasias in 15, Crohn's disease in 9 and ulcerative colitis in other 8 cases (Table IV).
See pictures.
Discussion
The CCE must be considered as a new technique to explore colon mucosa. It is a non-invasive and easy tool to manage so it is an advantage compared to conventional colonoscopy. The main indication of the CCE is the colon cancer screening and in order to find out the sensitivity and specificity of the CCE compared to colonoscopy in screening colorectal cancer, some studies have been developed in colorectal cancer screening (5,6,8).
The most important study published about CCE is a prospective, multicenter study comparing capsule endoscopy with colonoscopy in a group of patients with known or suspected colonic disease in the detection of colorectal polyps or cancer (10). A total of 328 patients were included in the study. Sensitivity and specificity of CCE to detect polyps of 6 mm in size or larger were 64% and 84%, respectively. It is important to comment that of 19 cancers detected by colonoscopy, 14 were detected by capsule endoscopy.
These results are similar to the two European studies recently (5,6) published. In these studies, the sensitivity was 69% and 76%, specificity was 81% and 64%, the positive predictive value was 74% and 83% and the negative predictive was 78% and 54%, respectively, for polyp detection. Although our study does not evaluate sensitivity and specificity compared to colonoscopy for the detection of polyps, the results of our study for any findings are similar to the before mentioned studies. Perhaps the sensitivity is higher and the specificity is lower, because CCE is able to detect colonic findings that conventional colonoscopy misses. In Eliakim et al. (5) study, in four cases colonoscopy had to be repeated because CCE detected significant colonic findings and colonoscopy was negative. Even polyps larger than 6 mms were detected by CCE and colonoscopy had failed to detect them. Possibly, the fact that CCE takes images from both ends improves visualization of lesions that the colonoscopy misses. Nevertheless, the new second-generation colon capsule endoscopy will improve the visualization of colonic lesions. Sensitivity and specificity for detecting colorectal polyps appear to be very good, suggesting a potential for improved accuracy compared with the first-generation system, although further prospective and comparative studies are needed (10). Two metaanalysis has been recently published and confirm that CCE is a reasonable method for screening asymptomatic individuals for colorectal polyps. It may be particularly useful for patients with "incomplete" colonoscopy, those with contraindications for conventional colonoscopy, and those unwilling to undergo colonoscopy because of its perceived inconvenience and discomfort (11,12).
In Van Gossum et al. (8) study, the preparation was good or excellent in 72% of the patients. The grade of cleanliness was excellent or good in 65%-85% of the patients included in the European studies (5,6). In our study the grade of cleanliness was good or excellent in 65,6%. The preparation chosen was a mixture of those used in the European studies.
It appears that colon cleanliness significantly influences the sensitivity of capsule endoscopy. In the largest stud y (8) the sensitivity was significantly higher in the patients with good or excellent cleanliness compared to those with poor or fair cleanliness. The sensitivity and specificity for the detection of polyps (≥6 mm) in patients with good or excellent cleanliness was 75% and 84%, respectively, and for the detection of such polyps in patients with poor or fair cleanliness, the sensitivity and specificity were 42% and 84%, respectively. In a recent study (13) the exclusion of NaP booster from CCE preparation resulted into a clinically meaningful reduction of the capsule excretion rate that was only partially compensated by the PEG booster.
The CCE rate of complete excretion was 81%-92% in the previous studies, and collateral complications of the procedure did not show. In our study the rate is similar (93%) to the rate detected in the Van Gossum study. No adverse events were detected and all capsules worked properly although some failure has been described (14).
Another important point is to evaluate cost-effectiveness of CCE. Only one study (15) has been published for colon cancer screening indication, but there are no more studies for the rest of indications. In this paper the cost-effectiveness of capsule endoscopy was studied by a computer model based on a Markov process. When simulating an initial compliance to capsule endoscopy 30% better than colonoscopy, capsule endoscopy became the more effective and more cost-effective option. So it is concluded that the cost-effectiveness of capsule endoscopy depends mainly on its ability to improve compliance to CRC screening.
Although the main objective of the CCE must be colon cancer screening, CCE should be considered a new technique able to detect colonic lesions in patients who refuse conventional colonoscopy or patients with incomplete colonoscopy. Nevertheless, more studies must be done to improve the colon cleanliness and also to compare it to colonoscopy in other pathologies, such as inflammatory bowel diseases, diverticulosis or vascular lesions.
In our study some limitations can be found. First of all, not all the CCE studies are being compared to conventional colonoscopy, but we must say that all these patients refused the conventional colonoscopy. Secondly, the statistics have been calculated for all the colonic findings and perhaps it should have been calculated for each lesion (but there are not enough patients to do so).
Therefore, based on these data, we consider that CCE is an effective and sure technique for the detection of lesions in colon and it could be used in clinical practice in some cases (patients who refuse conventional colonoscopy or incomplete colonoscopy). Nevertheless, further studies with larger seria and cost-effectiveness analysis are needed to confirm these data.
References
1. Caunedo A, Rodríguez-Téllez M, García-Montes JM, Gómez-Rodríguez BJ, Guerrero J, Herrerías JM Jr, Pellicer F, Herrerías JM. Usefulness of capsule endoscopy in patients with suspected small bowel disease. Rev Esp Enferm Dig. 2004; 96(1):10-21. [ Links ]
2. Muñoz-Navas M. Capsule endoscopy. World J Gastroenterol. 2009; 15(13): 1584-6. [ Links ]
3. Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, Seidman EG, Cellier C, Murray J, de Franchis R, Rösch T, Lewis BS. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007; 39(10): 895-909. [ Links ]
4. Pérez Roldán F, González Carro P, Legaz Huidobro ML, Roncero García-Escribano O, Ynfante Ferrús M, Aoufi S, Sánchez-Manjavacas Muñoz N, Ruiz Carrillo F. Efficacy of pediatric colonoscopy used as push enteroscopy in the management of capsule endoscopy findings. Rev Esp Enferm Dig. 2009; 101: 468-76. [ Links ]
5. Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006; 38(10): 963-70. [ Links ]
6. Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006; 38(10): 971-7. [ Links ]
7. Fernández-Urien I, Carretero C, Borda A, Muñoz-Navas M. Colon capsule endoscopy. World J Gastroenterol. 2008; 14(34): 5265-8. [ Links ]
8. Van Gossum A, Navas MM, Fernández-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, Costamagna G, Riccioni ME, Spada C, Petruzziello L, Fraser C, Postgate A, Fitzpatrick A, Hagenmuller F, Keuchel M, Schoofs N, Devière J. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009; 361(3): 264-70. [ Links ]
9. Lewis B, Rex D, Lieberman D. Capsule Colonoscopy-An Interim Report of a Pilot 3 Arm, Blinded Trial of Capsule Colonoscopy, Virtual Colonoscopy and Colonoscopy. Am J Gastroenterol 2006:101 (s2), S545-S561 (A1470). [ Links ]
10. Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, Kopelman Y, Adler SN. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009; 41(12): 1026-31. [ Links ]
11. Rokkas T, Papaxoinis K, Triantafyllou K, Ladas SD. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc. 2010; 71(4): 792-8. [ Links ]
12. Spada C, Hassan C, Marmo R, Petruzziello L, Riccioni ME, Zullo A, Cesaro P, Pilz J, Costamagna G. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin Gastroenterol Hepatol. 2010; 8(6): 516-22. [ Links ]
13. Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, Costamagna G. PillCam Colon Capsule Endoscopy: A Prospective, Randomized Trial Comparing Two Regimens of Preparation. J Clin Gastroenterol. 2011; 45(2): 119-24. [ Links ]
14. Simultaneous failure of Pillcam Colonth . N. Almeida, P. Figueiredo P. Freire, S. Lopes, F. Portela, M. Ferreira, Hermano Gouveia and M. Correira Leitiño. Rev Esp Enferm Dig. 2008; 100(11): 731-2. [ Links ]
15. Hassan C, Zullo A, Winn S, Morini S. Cost-effectiveness of capsule endoscopy in screening for colorectal cancer. Endoscopy. 2008; 40 (5): 414-21. [ Links ]
![]() Correspondence:
Correspondence:
J. M. Herrerías-Gutiérrez.
Servicio de Digestivo.
Hospital Universitario Virgen Macarena.
Avda. Dr. Fedriani s/n. Sevilla.
e-mail: jmhg@us.es
Received: 26-04-10.
Accepted: 31-08-10.