Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.103 no.5 Madrid may. 2011
https://dx.doi.org/10.4321/S1130-01082011000500002
Prospective validation of two models for ultrasonographic diagnosis of cirrhosis
Validación prospectiva de dos modelos de diagnóstico de cirrosis hepática mediante ultrasonografía
Manuel Alberto Macías-Rodríguez, Paloma Rendón-Unceta, María Teresa Ramos-Clemente-Romero, Luis Manuel Troiteiro-Carrasco and María Dolores Serrano-León
Deparment of "Gestión Clínica" of Digestive Diseases. Hospital Universitario Puerta del Mar. Cádiz, Spain
ABSTRACT
Objective: to perform a prospective validation and comparative analysis of two ultrasonographic diagnostic scores of cirrhosis in patients with silent liver disease.
Design: cross-sectional study, prospective and blind. ROC curves evaluated the diagnostic utility of: a) Bologna score (BS): assessment of liver surface nodularity and portal flow velocity, and b) Cadiz score (CS): assessment of liver echostructure, portal vein caliber and spleen area. Liver biopsy was considered the gold standard for the diagnosis of cirrhosis.
Patients: one hundred and thirteen patients, 76 men and 37 women, mean age 44 years old (range 18-73 years) referred for evaluation of chronic liver disease without clinical or biochemical evidence of advanced disease (absence of jaundice, ascites, encephalopathy, malnutrition or coagulopathy).
Results: cirrhosis was diagnosed in 25 patients (22.1%). BS: sensitivity 84%, specificity 79.5%, area under the ROC curve 86.7%. CS: sensitivity 84%, specificity 89.8%, area under the ROC curve 92.4%. Portal vein was not displayed in 7 patients (6%) and portal flow velocity was not recorded in 13 (11.5%). These results agree with those obtained in the original articles developing both scores. There were no statistically significant differences between the two scores. Specificity reached 97% with joint use of both models, but sensitivity decreased to 72%.
Conclusions: presence or absence of cirrhosis in patients with silent liver disease can be established by Doppler ultrasound with high diagnostic accuracy. The joint use of both scores has high diagnostic specificity. Both diagnostic models are highly reproducible.
Key words: Chronic hepatitis. Liver cirrhosis. Portal hypertension. Ultrasonography. Doppler ultrasound.
RESUMEN
Objetivo: realizar una validación prospectiva y un análisis comparativo de dos escalas de diagnóstico ecográfico de cirrosis en pacientes con enfermedad hepática silente.
Diseño experimental: estudio transversal, prospectivo y ciego. Mediante curvas ROC se evaluó la utilidad diagnóstica de: a) escala de Bolonia (EB): valoración de la nodularidad de la superficie hepática y la velocidad de flujo portal; y b) escala de Cádiz (EC): valoración de la ecoestructura hepática, calibre portal y área esplénica. La biopsia hepática se consideró el estándar para el diagnóstico de cirrosis.
Pacientes: 113 pacientes, 76 varones y 37 mujeres con edad media de 44 años (18-73) remitidos para estudio de una hepatopatía crónica sin indicios clínicos ni bioquímicos de enfermedad avanzada (ausencia de ictericia, ascitis, encefalopatía, malnutrición ni coagulopatía, actuales ni previas).
Resultados: veinticinco pacientes (22,1%) fueron diagnosticados de cirrosis. EB: sensibilidad 84%, especificidad 79,5%, área bajo la curva ROC 86,7%. EC: sensibilidad 84%, especificidad 89,8%, área bajo la curva ROC 92,4%. La vena porta no se visualizó en 7 pacientes (6%) y la velocidad de flujo portal no se determinó en 13 (11,5%). Estos resultados son superponibles a los obtenidos en los artículos originales. No se encontraron diferencias estadísticamente significativas entre ambas escalas. El empleo conjunto de los dos modelos incrementó la especificidad diagnóstica hasta el 97% con una sensibilidad del 72%.
Conclusiones: la presencia o ausencia de cirrosis en pacientes con enfermedad hepática silente puede ser establecida mediante ecografía con una elevada precisión diagnóstica. El empleo conjunto de ambas escalas alcanza una elevada especificidad diagnóstica. La reproducibilidad de ambas escalas es excelente.
Palabras clave: Hepatitis crónica. Cirrosis hepática. Hipertensión portal. Ultrasonografía. Ultrasonografía Doppler.
Introduction
The diagnosis of cirrhosis is a turning point in the follow-up of patients with chronic liver disease, since it determines the requirement for monitoring the occurrence of hepatocellular carcinoma or complications of portal hypertension. Liver biopsy has traditionally been considered the gold standard in the diagnosis of cirrhosis (1); however, although it is a safe technique in experienced hands, complications occur in about 3% of cases, with a mortality risk of 0.01-0.03% (2). In addition, liver biopsy may give rise to false negative results for cirrhosis, that have been described in 24-50% of cases, and staging variability between biopsy specimens of the same liver can reach 50% (5,6).
Currently, serological markers of fibrosis and transient elastography are widely used in lack of specific guidelines to establish the presence or absence of significant fibrosis and cirrhosis in patients with chronic liver disease. However, ultrasonography is the first imaging technique in the assessment of patients with suspected liver disease, and sonographic signs suggestive of cirrhosis have been widely described in the literature, usually in patients with clinically advanced disease. Conversely, the ability of ultrasonography in patients without clinical and biochemical data of advanced cirrhosis has rarely been studied, and conclusions have been discordant. To date, only Gaiani et al. (7) analyzed this situation in a study with external validation of its results: they achieved an 80% diagnostic accuracy in the diagnosis of cirrhosis based on the demonstration of liver surface irregularity and reduction of portal flow velocity.
In a prospective and blind study evaluating patients with silent chronic liver disease referred for liver biopsy, our group assessed the usefulness of 17 ultrasonographic variables in the diagnosis of cirrhosis. We concluded that a model including liver echoestructure, portal vein diameter and spleen area reached a diagnostic accuracy of 89% (8).
The aims of the present study have been: a) to analyze the external validity of the results in studies of Gaiani et al. and Macias et al.; and b) to compare the accuracy of both models in the diagnosis of liver cirrhosis.
Method
Patients
One hundred and thirteen consecutive patients with chronic liver disease, admitted for liver biopsy, were included in this prospective study. They were 76 men and 37 women, with ages comprised between 18 and 73 years old (median 44 years). All of them fulfilled the following inclusion criteria: elevated liver enzymes for more than 6 months in the absence of data of advanced liver disease (at the time of enrollment or in background) as ascites, encephalopathy, malnutrition, hyperbilirubinemia, hypoalbuminemia, coagulation disorders or evidence of portal hypertension (collateral circulation at physical examination or evidence of the presence of esophageal varices or hypertensive gastropathy).
The etiology of the liver disease was hepatitis C infection in 70 patients, viral B and C infection in 30, hepatitis B in 2, autoimmune hepatitis in 2, and alcoholism in 1 (it was unknown in 8 patients).
Echo Doppler study
All examinations were performed just before liver biopsy by one of two experienced operators with joint training (M.M.R and P.R.U.) without knowledge of patient characteristics. The exploration was performed with a Hitachi EUB 6500 equipment with 2.5-5 MHz multifrequency probe equipped with pulsed and color Doppler module (Hitachi Medical Corporation, Tokyo, Japan) after an overnight fast and 15 minutes in supine position.
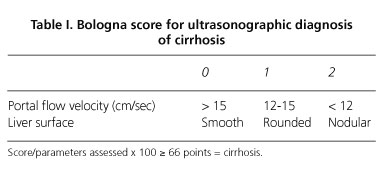
Assessed parameters were the 5 included in the models of Gaiani et al. (Bologna score) (7) and Macias et al. (Cadiz score) (8), using the same methodology and cutoff values expressed in the original studies (Tables I and II). In summary: a) liver surface was scanned at the interface with the kidney and gallbladder, posterior edge of the left lobe and in the position closest to the transducer; b) flow velocity was determined in the extrahepatic portal vein at the junction with the hepatic artery; c) liver parenchyma was evaluated in both lobes; d) portal vein caliber was measured in the same place where the flow rate was determined; and e) spleen area was established from its perimeter determined manually at maximum longitudinal section. Numerical parameters were the result of the arithmetic mean of three consecutive recordings. The chosen sample volume for Doppler measurements was two third of the vessel diameter and the incidence angle of the Doppler beam was between 30 and 60 degrees.
Histological diagnosis
Percutaneous liver biopsy was performed with tru-cut 16-G needle, the ultrasound diagnosis being unknown for the pathologist. Fibrosis was graded according to the METAVIR score. Cirrhosis was defined by the presence of well-defined nodules of hepatocytes surrounded by fibrous tissue, in a sample of at least 15 mm in length showing at least 11 portal tracts (9). Informed consent for the accomplishment of the test was obtained from each patient.
Statistical analysis
Numerical variables are expressed as mean ± standard deviation, categorical variables as number of cases. Accuracy of both diagnostic models for cirrhosis was compared by the homogeneity test of the areas under ROC curves with a confidence interval of 95%. Differences were considered significant if p < 0.05. Data were analyzed by statistical packages EPIDAT 3.1 and SPSS 11.5.
Results
A final diagnosis of cirrhosis was demonstrated in 25 of the 113 patient (22%). Metavir score in the remaining 88 patients was F0, 33; F1, 36; F2, 17; and F3, 2 patients. There were no differences between groups regarding age (50,1 ± 8,9 vs. 42,6 ± 8,6, p = 0.72), platelet count (133,166 ± 90,177 vs. 196,977 ± 65,386, p = 0.22), or etiology of the disease (cirrhosis was due to hepatitis C in 14 patients, HIV and C co-infection in 7, and ethanol, hepatitis B, autoimmune disease and cryptogenic in the remaining 4 patients).
All of the analyzed variables, except for portal vein flow velocity, showed statistical differences between cirrhotic and non-cirrhotic patients (Table III). Irregular liver surface, coarse liver parenchyma, and portal vein diameter were very specific parameters, but only surface irregularity showed sensitivity above 70% in the diagnosis of cirrhosis. Bowel gas made not possible the assessment of portal vein caliber in seven patients as well as flow velocity in 13.
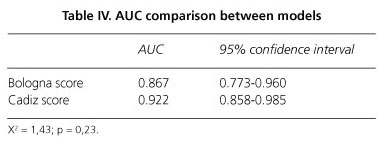
By the model of Gaiani et al., diagnosis of cirrhosis was established with sensitivity of 84%, specificity of 79.5% and diagnostic accuracy of 86.7%. The model of Macias et al. also found sensitivity of 84%, but specificity increased to 89.8% and diagnostic accuracy reached 92% (Fig. 1). Differences observed between both models were not statistically significant (Table IV).
Doppler ultrasonography gave rise to false diagnoses of cirrhosis in 24 patients: 9 cases using the model of Macias et al. and 18 with the one of Gaiani et al. Specificity increases to 97% when both models agree in the diagnosis of cirrhosis, but sensitivity decreases to 72% and AUC fits not better than our model. Only three patients were over staged with this strategy, but esophageal varices were demonstrated in one of them one year later, so making correct ultrasonographic diagnosis.
Discussion
Doppler ultrasonography supports, like other imaging techniques, the important limitation of variability between different equipments, operators, and patient groups, making not possible the immediate generalization of results obtained in local studies. Conversely, an external validation of results is necessary, but this is not usually performed. The usefulness of Doppler ultrasonography in the diagnosis of cirrhosis has been assessed in multiple studies, but sonographic signs traditionally considered suggestive of cirrhosis lack a proper definition of the patient population whom they are applicable to, as well as an external validation of their usefulness for different operators.
On the other hand, the emergence of laboratory tests indicating the presence and quantity of hepatic fibrosis and transient elastography has displaced the interest for Doppler ultrasonography in the staging of chronic liver disease. Nevertheless, patients with abnormalities in the liver blood tests are systematically subjected to ultrasonography. These patients frequently lack clinical or biochemical signs of severe disease, and the etiology is unknown at the moment of the exam. Even in this situation, it has been shown that 30% of patients with viral liver disease already have cirrhosis (7,8). This clinical scenario is different to that described in studies analyzing the applicability of serological markers of fibrosis or transient elastography, mostly including patients followed up by a viral liver disease for evaluation of their therapeutic needs.
It is known that Doppler ultrasound lacks the ability to evaluate intermediate stages of hepatic fibrosis, but its utility for determining the existence of liver cirrhosis in patients without clinical or biochemical tests of advanced disease has been analyzed in well designed prospective and blind studies (7,8,10,11). However, only one of them included an external validation of results, although restricted to a different study group explored by the same operators and equipments: Gaiani et al. (7), analyzed the usefulness of 7 Doppler ultrasound parameters in the diagnosis of liver disease (liver size, size ratio between liver lobes, abnormalities in liver parenchyma, liver surface irregularity, caliber and flow velocity in the portal vein, and spleen size) finally developing an equation that required only two of them (liver surface irregularity and portal flow velocity) to establish the presence or absence of cirrhosis with sensitivity of 78%, specificity 80% and diagnostic accuracy of 80%. The equation can be calculated even if one of the parameters is not defined. This is an important consideration since measurement of portal flow velocity is sometimes difficult to assess (6-13%) by intestinal bloating.
Our previously published study (8) has been the only one analyzing the usefulness of all of the Doppler sonographic parameters related to cirrhosis in the literature, including those contained in the score of Gaiani et al. (7). In spite of similarities in patient's characteristics, none of the Bologna score parameters entered in the model showing the best diagnostic accuracy in that study. This discrepancy, besides the need of validating our original results, led us to develop the current study.
In the present study, both scores have been useful in the diagnosis of cirrhosis in patients with silent liver disease. Histological specimen showed cirrhosis in 22% of patients. Our diagnostic model reached better specificity and accuracy, but differences were not statistically significant. We could not measure portal vein caliber in seven patients, but score obtained with the other two parameters in the model also concurred with the histological diagnosis (data not shown). We were not able either to measure portal venous flow velocity in 13 patients but, as previously stated, the score of Gaiani et al. (7) allows its application with the only evaluation of liver surface. It is our opinion that the capability to diagnose cirrhosis based only in this parameter may be limited, since it is often difficult to ensure whether that is smooth or irregular. Operator can also be influenced in definition of liver surface by the presence of other ultrasonographic signs of liver disease such as abnormalities in liver parenchyma, portal vein dilation, enlarged spleen or collateral circulation. On the other hand, our model may be some subjective in the assessment of liver echostructure (12). This is because, unless it is really nodular, a finding frequently accompanied by visualization of an irregular surface, abnormalities in liver surface should be accompanied by other highly objective signs such as portal vein dilation or spleen enlargement to establish the diagnosis of cirrhosis.
Taking liver biopsy as gold standard, false diagnoses of cirrhosis with Doppler ultrasound occur with both diagnostic scores. Nevertheless, it is important to recognize that one third to one forth of them are truly cirrhosis, being actually false negative results of biopsy, as demonstrated by the development of clinical and endoscopic data of cirrhosis with portal hypertension after short monitoring (7,8). Considering liver biopsy as gold standard for diagnosing cirrhosis is questioned also by the presence of sampling variability between biopsies in the same patient and by significant interobserver disagreement, which reach 25% even with 25 mm samples (13). False ultrasonographic diagnosis of cirrhosis in our present study was reduced from 24 to 2 patients by the joint use of both scores, but this strategy diminished sensitivity to 72%.
Our study does not dismiss the utility of other Doppler ultrasound parameters in the diagnosis of cirrhosis: caudate lobe hypertrophy, splenic vein dilation (and absence of variations in its caliber with respiratory movements), flattening of hepatic vein flow wave or presence of collateral circulation all have adequate specificity in the appropriate clinical setting but their sensitivities do not exceed 50%. In the same way, other authors have used other scores with good results in patients with more advanced disease (14-20).
The appearance of new technologies in the last years has extended the spectrum of possibilities of ultrasonography for the diagnosis of cirrhosis; so, time from venous injection of an ultrasound contrast agent to its visualization in the portal and hepatic veins (the called transit time) is different when comparing cirrhotic and non-cirrhotic liver disease (21,22). Furthermore, ultrasonography can be complementary to fibroscan and other noninvasive techniques as reported by Berzigotti et al. (23), whom suggest the superiority of joint assessment of liver elasticity using fibroscan and nodularity of liver surface by ultrasound in the diagnosis of cirrhosis over each of the techniques. Authors applied a computerized analysis technique to ultrasound images in patients with advanced liver disease, which prevent the extrapolation of their results. This study especially stand out the value of subjective evaluation of surface irregularity in the diagnosis and exclusion of cirrhosis compared to computerized analysis (the last was useful in indeterminate cases) and the greater applicability of ultrasound over transient elastography. This is especially important in the study of ascites of unknown origin, for which fibroscan is not useful.
In conclusion, the role of liver biopsy as gold standard in the diagnosis of liver cirrhosis is a questionable fact. Being Doppler ultrasound a technique of generalized application in patients with suspected liver disease, operators should be aware of its high capacity to assert or exclude the diagnosis of liver cirrhosis and to know the utility of different ultrasound parameters and diagnostic scores. Finally, it seems reasonable to analyze the joint utility of noninvasive fibrosis scales, transitional elastography and Doppler ultrasound (including the latest technological advances) in the diagnosis of liver cirrhosis.
References
1. Bruguera M, Borda J, Mass P, Rodes J. A comparison of the accuracy of peritoneoscopy and liver biopsy in the diagnosis of cirrhosis. Gut 1974;15:799-800. [ Links ]
2. García-Tsao G, Boyer J. Outpatient liver biopsy: how safe is it? Ann Intern Med 1993;118:150-3. [ Links ]
3. Nord H. Biopsy diagnosis of cirrhosis: blind percutaneous versus guided direct vision techniques-a review. Gastrointest Endosc 1982; 28:102-4. [ Links ]
4. Soloway R, Baggenstoss A, Schonenfield L, Summerskill W. Observer error and sampling variability tested in evaluation of hepatitis and cirrhosis by liver biopsy. Dig Dis Sci 1971;16:1082-6. [ Links ]
5. Pagliaro L, Rinaldi F, Craxi A, Di Piazza S, Filippazzo G, Gatto G, et al. Percutaneous blind biopsy versus laparoscopy with guided biopsy in diagnosis of cirrhosis. A prospective, randomised trial. Dig Dis Sci 1983;28:39-43. [ Links ]
6. Maharaj B, Maharaj R, Leary W, Cooppan R, Naran A, Pirie D, et al. Sampling variability and its influence on the diagnosis yield of percutaneous liver biopsy of the liver. Lancet 1986;i:523-5. [ Links ]
7. Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D'Errico A, et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol 1997;27:979-85. [ Links ]
8. Macías-Rodríguez MA, Rendón-Unceta P, Navas-Relinque C, Tejada-Cabrera M, Infantes-Hernández JM, Martín-Herrera L. Ultrasonography in patients with chronic liver disease: its usefulness in the diagnosis of cirrhosis. Rev Esp Enferm Dig 2003;95:251-7. [ Links ]
9. Bedossa P. The French METAVIR cooperative study group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15-20. [ Links ]
10. Lin D, Sheen I, Chiu C, Lin S, Kuo Y, Liaw Y. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound 1993;21:303-8. [ Links ]
11. Cioni G, Tincani E, D'Alimonte P, Cristani A, Ventura P, Abbato G, et al. Relevance of reduced portal flow velocity, low platelet count and enlarged spleen diameter in the non-invasive diagnosis of compensated liver cirrhosis. Eur J Med 1993;2:408-10. [ Links ]
12. Varas Lorenzo M. Ultrasonography and liver cirrhosis. Rev Esp Enferm Dig 2003;95:245-7. [ Links ]
13. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-57. [ Links ]
14. Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999;30:472-8. [ Links ]
15. Harbin W, Robert N, Ferrucci J. Diagnosis of cirrhosis based on regional changes in hepatic morphology. Radiology 1980;135:273-83. [ Links ]
16. Giorgio A, Amoroso P, Lettieri G, Fico P, de Stefano G, Finelli L, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986;161:443-5. [ Links ]
17. Hess F, Schmiedl U, Koelbel G, Knecht R, Kurtz B. Diagnosis of the liver cirrhosis with US: receiver-operating characteristic analysis of multidimensional caudate lobe indexes. Radiology 1989;171:349-51. [ Links ]
18. Goyal A, Pokharna D, Sharma S. Ultrasonic diagnosis of cirrhosis: reference to quantitative measurements of hepatic dimensions. Gastrointest Radiol 1990;15:32-4. [ Links ]
19. Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989;172: 389-92. [ Links ]
20. Colli A, Cocciolo M, Riva C, Martinez E, Prisco A, Pirola M, et al. Abnormalities of Doppler waveform of the hepatic veins in patients with chronic liver disease: correlation with histologic findings. AJR 1994;162:833-7. [ Links ]
21. Lim AK, Taylor-Robinson SD, Patel N, Eckersley RJ, Goldin RD, Hamilton G, et al. Hepatic vein transit times using a microbubble agent can predict disease severity non-invasively in patients with hepatitis C. Gut 2005;54:128-33. [ Links ]
22. Staub F, Tournoux-Facon C, Roumy J, Chaigneau C, Morichaut-Beauchant M, Levillain P, et al. Liver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoring. Eur Radiol 2009;19:1991-7. [ Links ]
23. Berzigotti A, Abraldes J, Tandon P, Erice E, Gilabert R, García-Pagan J, et al. Ultrasonographic evaluation of liver surface and transient elastography in clinically doubtful cirrhosis. J Hepatol 2010;52:846-53. [ Links ]
![]() Correspondence:
Correspondence:
Manuel Alberto Macías Rodríguez.
Unidad de Gestión Clínica de Aparato Digestivo.
Hospital Universitario Puerta del Mar.
Avda. Ana de Viya, 21.
11009 Cádiz. Spain.
e-mail: mmacias@comcadiz.com
Received: 13-09-10.
Accepted: 26-01-10.











 texto en
texto en