My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.104 n.4 Madrid Apr. 2012
https://dx.doi.org/10.4321/S1130-01082012000400005
Laparoscopic versus open transhiatal esophagectomy for distal and junction cancer
Esofagectomía laparoscópica frente a abierta en el cáncer esofágico distal y de la unión
Kirsten W. Maas, Surya S.A.Y. Biere, Joris J.G. Scheepers, Suzanne S. Gisbertz, Donald L. van der Peet and Miguel A. Cuesta
Department of Surgery. VU University Medical Centre. Amsterdam, Netherlands
ABSTRACT
Background: the only curative treatment for esophageal cancer is surgical resection. This treatment is associated with a high morbidity rate and long in-hospital recovery period. Both transthoracic and transhiatal esophagectomies are performed worldwide. The transhiatal approach may reduce the respiratory infection rate in compromised patients with distal esophageal and gastro-esophageal (GE) cancers. Minimally invasive esophagectomy could further improve post-operative outcome. Two cohorts of laparoscopic and open transhiatal esophagectomy for cancer were compared for short- and long-term outcome.
Methods: from January 2001 through December 2004, 50 patients who underwent laparoscopic transhiatal esophagectomy were compared to a historical group of 50 patients who had undergone open transhiatal esophagectomy between January 1998 and December 2000. Post-operative management was identical in both groups.
Results: no significant differences were seen between the two groups with regard to baseline characteristics and oncological parameters including resection margin (R0 82 vs. 74%, p = 0.334) and 5-year survival. Operation time did not differ significantly between the groups. (300 vs. 280 min, p = 0.110). Median hospital stay and intensive care unit stay were significantly shorter in the laparoscopic group (13 vs. 16 days, p = 0.001 and 1 vs. 3 days, p = 0.000 respectively).
Conclusion: minimally invasive transhiatal esophagectomy is feasible and has the same oncological outcome as open transhiatal esophagectomy. Faster recovery without a significant longer operation time could be the major benefit of the laparoscopic transhiatal approach. To our knowledge, this is the largest comparative study in literature comparing laparoscopic transhiatal with open transhiatal esophagectomy for cancers of distal and GE junction. Randomized trials are needed to further clarify the role of laparoscopic transhiatal approach for esophageal cancer.
Key words: Esophageal cancer. Transhiatal esophageal resection. Laparascopic trasnhiatal resection. Morbidity of esophageal resection.
Introduction
The incidence of adenocarcinoma of the esophagus and gastro-esophageal (GE) junction is rapidly rising (1,2). The only curative therapy remains surgery. For years, the procedure of choice for esophageal cancer was the Ivor-Lewis operation, later modified by McKeown (3). With this modified procedure, the esophagus is resected by means of a right-sided thoracotomy combined with a laparotomy using cervical esophagogastric anastomosis (3,4). The other frequently used procedure is the transhiatal esophageal resection according to Orringer in which a thoracotomy is avoided (5). Both procedures have high complication rates, varying from 40 to 80%, and the in-hospital mortality rate averages 7.5% to less than 5% in experienced centers (6). A meta-analysis that compared transthoracic and transhiatal resections concluded that although transthoracic resections had significantly higher pulmonary morbidity and mortality rates, 5 year survival was about 20% after both approaches (7).
The approach and extent of the resection that is necessary is still controversial. In a prospective randomized study by Hulscher et al. transthoracic esophageal resection with systematic abdominal and mediastinal lymph node dissection (two-field lymphadenectomy) were compared with the classic transhiatal approach (8). The transhiatal approach had a lower morbidity than the extended lymphadenectomy. Even if a trend was observed with an advantage for the transthoracic approach in tumors located in the mid-esophagus, the median survival, disease-free, and quality-adjusted survival for the most common lower esophageal cancers were not statistically significant (8). Since postoperative morbidity after esophagectomy in general is increased in patients > 75 years of age, with prehospital stay higher than 20 days and with prior respiratory disease, the transhiatal route may be beneficial for these compromised patients (9).
A meta-analysis showed that minimally invasive esophagectomy could lower morbidity and shorten hospital stay (10). This effect was only present for minimally invasive transthoracic esophagectomy as the case-control studies reporting on laparoscopic transhiatal esophagectomy had a small sample size. To date no randomized trial has been performed comparing laparoscopic and open transhiatal esophagectomy.
This study compares the short- and long-term results of two cohorts of 50 consecutive patients with cancer of the distal esophageal and GE junction who were approached by a minimally invasive procedure or an open procedure. To our knowledge, this is the largest case-control study in literature comparing laparoscopic transhiatal esophagectomy with open transhiatal resection (10).
Patients and methods
From January 2001 through December 2004, fifty consecutive patients who underwent laparoscopically assisted transhiatal esophageal resection in the VU university medical center were prospectively followed. The results were compared with an unselected historical group of fifty consecutive patients who underwent an open transhiatal esophageal resection in the VU university medical center in the pre-laparoscopic period of January 1998 through December 2000. All patients presented with a squamous cell carcinoma or an adenocarcinoma of the distal 5 centimeters of the esophagus or the GE junction.
Patients with previous upper abdominal surgery did not undergo a laparoscopic approach. Patients with a colon interposition were excluded for the analysis. Pre-operative staging was performed by means of endoscopic ultrasound, computed tomography (CT) -scan of thorax and abdomen and a neck ultrasound.
After May 1999, some patients with locally advanced esophageal cancer (T3, N0, N1) received neoadjuvant chemo-immunotherapy (cisplatin, gemcitabine plus GM-CSF).
Operative technique
The laparoscopic transhiatal esophagectomy was described in an earlier publication by Scheepers et al. (11). In summary the patient is operated in supine position with neck extended with exposure of the right side. The operating surgeon is standing between the legs with two assistants on both sides. Five trocars are placed in the upper abdomen. A transhiatal dissection of the esophagus is laparoscopically performed in the plane between the pericardium, aorta and both pleurae. Anteriorly, dissection is performed in an avascular plane in the anterior mediastinum with visualization of the pericardium and the pulmonary vein up to the lymph nodes located in the carina. Posteriorly the aorta is approached at the level of the hiatus and in an avascular plane dissected free as high as possible in the posterior mediastinum. Lateral dissection is performed on both sides at the level of the pleurae, which are always opened and en bloc resection is performed when needed. Next the stomach is mobilized including a lymphadenectomy of the celiac trunk. The next step is dissection of the cervical esophagus by a second surgeon and in the meantime introduction of a HandPort system (Smith & Nephew, Inc, Andover, MA) is performed through a 7 cm periumbilical incision. A venous stripper is introduced into the gastric lumen by a small incision in the lesser curvature and then pushed to the cervical esophagus. The cervical esophagus can now be divided and with the hand of the first surgeon in the abdomen and under laparoscopic vision controlled stripping can be safely performed. After retrieval of the specimen the mobilization of the stomach is completed and a small gastric conduit is created by using the 90 mm GIA (US Surgical, Norwalk, CT) stapling device. The gastric tube is oversewn and attached to the nasogastric tube, replaced in the abdomen and pushed into the cervical esophagus under vision. A hand sewn end-to-side cervical anastomosis is then performed.
An identical procedure described by Orringer and Sloan (6) was performed in the patients who underwent an open transhiatal esophageal resection. A pyloroplasty was performed only in the first 14 patients.
Post-operative management
Post-operatively, patients were ventilated mechanically at the intensive care unit (ICU) and extubated when haemodinamically and respiratory stable. Extubated patients were admitted to the medium care unit (MCU) and from there to the regular ward. Patients were fed through the jejunostomy feeding tube from the first day after their operation until the oral feeding could be completely resumed. On post-operative day 5, a swallow X-ray examination was performed to assess the anastomosis and gastric tube passage. When no leakage and a good passage were seen, the nasogastric tube was removed and oral feeding was started. Patients were discharged when they were completely mobile and able to feed themselves orally.
Statistical analysis
Statistical analysis was performed using the SPSS software package (SPSS, Chicago, IL, USA). Medians and interquartile ranges at the 25th and 75th percentile were calculated and subsequently depicted when relevant. Mann-Whitney U tests and Chi-square tests were used when appropriate. Survival curves were obtained using the Kaplan-Meier method. Survival of both groups was compared with the log-rank test. Significance was set at p < 0.05.
Results
Between January 2001 and December 2004, fifty consecutive patients with a squamous cell carcinoma or an adenocarcinoma of the distal esophagus or GE junction underwent laparoscopic transhiatal esophageal resection. The results were compared with the results of the group of fifty consecutive patients with tumors at the same localization who underwent a conventional open transhiatal esophageal resection in the pre-laparoscopy period between January 1998 and December 2000.
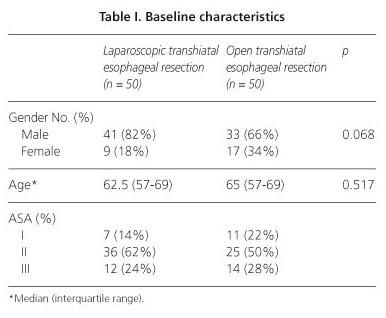
There were no significant differences between the groups in terms of gender, age, and American Society of Anesthesiologists (ASA) distribution (Table I). Fourteen patients (28%) in the open group and no patients in the laparoscopic group underwent a pyloroplasty (p = 0.000). Moreover, a significant difference was observed between the laparoscopic and open groups in the number of patients who received neoadjuvant chemotherapy (23 vs. 13, p = 0.037). Nine laparoscopically assisted operations (18%) were converted to open procedures. The reasons for conversions are depicted in table II. Laparoscopic mediastinal dissection of the esophagus could be accomplished in 44 patients (88%).
Tumor characteristics are listed in table III. There are no important differences between the groups in terms of the histological type of tumor, TNM stage, tumor localization and tumor differentiation. Tumor-free margins were obtained in 41 (82%) of the 50 patients who underwent laparoscopic resection and in 37 patients (74%) after open resection. R1 resections were found in 9 (18%) and 13 (26%) respectively, with microscopic margins at the distal part of the specimen. No R2 resections were carried out in both groups. No significant difference was found between the two groups regarding radicality of resection (p = 0.334). The median number of harvested lymph nodes was 14 (interquartile range: 10-19) in the laparoscopic group and 11 (interquartile range: 8-15) in the open group (p = 0.754).
The median operation time was longer in the laparoscopic group (300 minutes, interquartile range: 265-320) than in the open procedure (280 minutes, interquartile range: 250-320) but the difference was not significant (p = 0.110). Median blood loss was less in the laparoscopic group (500 ml, interquartile range: 400-650) compared with the open group (900 ml, interquartile range: 650-1,400) (p = 0.000). Median post-operative ICU stay was longer in the open group (3.0 days, interquartile range: 2.0-4.0) vs. the laparoscopic group (1.0 days, interquartile range: 1.0-2.0) (p = 0.000). The median hospital stay was 13 days (interquartile range: 11-16) in the laparoscopic and 16 days (interquartile range: 14-20) in the open group (p = 0.001) (Table IV).
Morbidity and mortality
No hospital mortality was recorded for the laparoscopic group, and one patient died after an open procedure (2%) due to acute respiratory distress syndrome. The morbidity rate was comparable in the laparoscopic (42%) and open (66%) group (Table V).
Pulmonary (i.e. respiratory infections) and cardiac complications were seen less often in the laparoscopic group than in the open group (12 vs. 19), however not statistically significant (p = 0.130). The re-operation rate was 2 (4%) and 3 (6%) in the laparoscopic and open group respectively (p = 0.646).
Survival
Kaplan-Meier analysis at 36 months showed an overall survival of 36% (95% confidence interval: 16.1-55.9) for the laparoscopic group and 38.3% (95% confidence interval: 24.4-52.2) for the open group. At five years overall survival was 29% for the laparoscopic group and 26% for the open group (Fig. 1). Median disease free survival at 36 months was 31% (95% confidence interval: 29.9-64.0) for the laparoscopic group versus 30.0% (95% confidence interval: 26.2-64.0) for the open group. For 5 years the disease free survival was 23% for laparoscopic and 21% for the open group. No statistical differences in mean survival and mean disease free survival were found after the cohorts were corrected for neoadjuvant therapy.
Discussion
To date both transthoracic and transhiatal esophagectomy are performed worldwide for distal esophageal or GE junction cancers. The objective of this study was to investigate, in the largest case-control study in literature, the role and feasibility of laparoscopic transhiatal eso-phagectomy.
Several minimally invasive approaches have been described to reduce operative trauma, improve dissection of the esophagus and tumor, reducing morbidity. The right thoracoscopic approach, the transhiatal approach, and microscopic endoscopic mediastinal dissection are already being performed (12-16). No randomized trials have been performed comparing laparoscopic transhiatal esophagectomy with an open resection.
In spite of initial high percentage of respiratory complication after thoracoscopic esophageal resection (14,17). The systematic standardization of the procedure by Luketich et al. (15) has demonstrated that the three-stage operation can be performed safely, in an acceptable operating time, with important advantages in the post-operative recovery of the patients and an oncological outcome at least as good as that after conventional surgery. In their experience of 222 patients, median ICU stay was 1 day and the hospital stay was 7 days, with an operative mortality of 1.4%. Major and minor complication rates were 32 and 23.9%, respectively. The same results are found by Nguyen et al. (18,19). The laparoscopic transhiatal approach has been performed in more limited number of patients by different authors (13,20,21). Conversion rates of 11.6% and morbidity rates of 30% have been described by Bonavina et al. in a series of 43 patients, but no comparative studies have been published (20). The results of the series presented here, concerning morbidity and mortality are consistent with the results published in the literature for both the laparoscopic and the open transhiatal approach (7,8,13,20-23). Furthermore, there are no differences concerning morbidity, mortality and operation time between the laparoscopic and open groups, but significantly less blood loss, shorter ICU stay and hospital stay was found in the laparoscopic transhiatal approach.
A point of consideration might be the conversion rate of 18%, this could be possible caused by the learning curve. Nevertheless, in 88% of the patients, the mediastinal dissection of the esophagus could be accomplished laparoscopically without anesthesiological hazards, especially in relation to the opening of the pleura that could produce a tension pneumothorax. Adaptation of positive end expiratory pressure and an increase of minute volume of the mechanical ventilation could avoid this problem and consequent conversion in all patients (24). Furthermore, laparoscopic transhiatal approach will permit perfect visualization of the mediastinal structures in relation to the tumor up to the carina, making this operation no longer a blind procedure, avoiding also the hemodynamic instability during the conventional dissection by the use of the retractor and manual dissection (25).
Retrieval of the tumor through a small well protected transumbilical incision instead of through a cervical incision may avoid the appearance of port-site metastases as in the case of laparoscopic colonic surgery for cancer. Moreover, once the specimen is retrieved, dissection around the pylorus and the origin of the gastroepiploic vessels, can be completed followed by formation of the gastric tube, using the conventional GIA-90. In this fashion the operation is time sparing and cost-effective.
The use of pyloroplasty remains controversial as well (17). Even though many authors still include the drainage of the pylorus in the operative procedure (15). In the current study, with the exception of the first open 14 operated patients, who underwent a routine pyloroplasty procedure, the avoidance of this pyloroplasty in the following patients did not lead to any emptying problems of the gastric tube during the post-operative period (26). Therefore, we do not recommend a routine pyloroplasty as part of the gastric tube formation. Interpreting the results of this study one has to consider the fact that the outcome of the 9 patients whom the laparoscopic procedure was converted to an open procedure, were analyzed within the laparoscopy group.
The laparoscopic transhiatal approach used in this study showed important advantages over the open approach, including less operative blood loss, shorter ICU stay, and shorter hospital stay with the same oncological outcome. This makes the laparoscopic transhiatal esophageal resection for tumors of the distal esophagus a feasible procedure. A randomized study would further clarify the role of a laparoscopic approach for distal esophageal cancer.
References
1. Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53. [ Links ]
2. Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology 1993;104:510-3. [ Links ]
3. Mckeown KC. Total 3-stage esophagectomy for cancer of the esophagus. Br J Surg 1976;63:259-62. [ Links ]
4. Lewis I. The surgical treatment of carcinoma of the oesophagus with special reference to a new operation for growths of the middle 3rd. Br J Surg 1946;34:18-31. [ Links ]
5. Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [ Links ]
6. Collard JM, Otte JB, Fiasse R, Laterre PF, De Kock M, Longueville J, et al. Skeletonizing en bloc esophagectomy for cancer. Ann Surg 2001;234:25-32. [ Links ]
7. Hulscher JBF, Tijssen JGP, Obertop H, van Lanschot JJB. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [ Links ]
8. Hulscher JBF, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus 3. N Engl J Med 2002;347:1662-9. [ Links ]
9. Martin-Perez E, Serrano PA, Figueroa JM, Clerigue A, Larranaga E. Carcinoma of the esophagus: risk factors of morbimortality following esophagectomy. Rev Esp Enferm Dig 1994;85:239-42. [ Links ]
10. Biere SSAY, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chirurgica 2009;64:121-33. [ Links ]
11. Scheepers JJ, Veenhof AA, van der Peet DL, van Groeningen C, Mulder C, Meijer S, et al. Laparoscopic transhiatal resection for malignancies of the distal esophagus: outcome of the first 50 resected patients. Surgery 2008;143:278-85. [ Links ]
12. Azagra JS, Ceuterick M, Goergen M, Jacobs D, Gilbart E, Zaouk G, et al. Thoracoscopy in esophagectomy for esophageal cancer. Br J Surg 1993;80:320-1. [ Links ]
13. DePaula AL, Hashiba K, Ferreira EAB, DePaula RA, Grecco E. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [ Links ]
14. Dallemagne B, Weerts JM, Jehaes C. Subtotal esophagectomy by thoracoscopy and laparoscopy. Minim Invasive Ther Allied Technol 1992;1:183-5. [ Links ]
15. Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, et al. Minimally invasive esophagectomy - outcomes in 222 patients. Ann Surg 2003;238: 486-94. [ Links ]
16. Buess G, Kaiser J, Manncke K, Walter DH, Bessell JR, Becker HD. Endoscopic microsurgical dissection of the esophagus (EMDE). Int Surg 1997;82:109-12. [ Links ]
17. Law S, Fok M, Chu KM, Wong J. Thoracoscopic esophagectomy for esophageal cancer. Surgery 1997;122:8-14. [ Links ]
18. Nguyen NT, Follette DM, Wolfe BM, Schneider PD, Roberts P, Goodnight JE Jr. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 2000;135:920-5. [ Links ]
19. Nguyen NT, Roberts P, Follette DM, Rivers R, Wolfe BM. Thoracoscopic and laparoscopic esophagectomy for benign and malignant disease: lessons learned from 46 consecutive procedures. J Am Coll Surg 2003;197:902-13. [ Links ]
20. Bonavina L, Bona D, Binyom PR, Peracchia A. A laparoscopy-assisted surgical approach to esophageal carcinoma. J Surg Res 2004; 17:52-7. [ Links ]
21. Van den Broek WT, Makay O, Berends FJ, Yuan JZ, Houdijk AP, Meijer S, et al. Laparoscopically assisted transhiatal resection for malignancies of the distal esophagus. Surg Endosc 2004;18:812-7. [ Links ]
22. Swanstrom LL, Hansen P. Laparoscopic total esophagectomy 3. Arch Surg 1997;132:943-7. [ Links ]
23. Serrano L, Sánchez JM, García I, Domínguez J. Diagnostic and therapeutic thoracoscopy in esophageal cancer. Rev Esp Enferm Dig 1997;89:79-81. [ Links ]
24. Makay O, van den Broek WT, Yuan JZ, Veerman DP, Helfferich DW, Cuesta MA. Anesthesiological hazards during laparoscopic transhiatal esophageal resection - A case control study of the laparoscopic-assisted vs. the conventional approach. Surg Endosc 2004;18:1263-7. [ Links ]
25. Pinotti HW, Zilberstein B, Pollara W, Raia A. Esophagectomy without thoracotomy. Surg Gynecol Obstet 1981;152:345-6. [ Links ]
26. Mannell A, Mcknight A, Esser JD. Role of pyloroplasty in the retrosternal stomach - results of a prospective, randomized, controlled trial. Br J Surg 1990;77:57-9. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Miguel A. Cuesta.
Department of Surgery.
VU University Medical Center.
De Boelelaan 1117.
1081 HV. Amsterdam. The Netherlands.
e-mail: ma.cuesta@vumc.nl
Received: 02-11-11.
Accepted: 19-12-11.