Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.104 no.5 Madrid may. 2012
https://dx.doi.org/10.4321/S1130-01082012000500014
LETTERS TO THE EDITOR
Recurrent hepatotoxicity associated with etanercept and adalimumab but not with infliximab in a patient with rheumatoid arthritis
Hepatotoxicidad recurrente asociada a etanercept y adalimumab pero no a infliximab en una paciente con artritis reumatoide
Key words: Etanercept. Adalimumab. Infliximab. Drug induced liver injury. Rheumatoid arthritis.
Palabras clave: Etanercept. Adalimumab. Infliximab. Alteración hepática inducida por medicamentos. Artritis reumatoide.
Dear Editor,
Tumour necrosis factor (TNF-α) antagonists are being commonly used in the treatment of rheumatological, dermatological, and gastroenterological autoimmune diseases. At present, there are five marketed TNF-α antagonists: infliximab, adalimumab, etanercept, golimumab and certolizumab. All five agents bind directly to TNF-α, threreby inhibiting its signalling (1). Acute and long-term systemic toxicity is a concern with these immunomodulators and liver injury is increasingly been described, particularly with infliximab yet causality assessment in the setting of patients with potential confusion factors including treatment with other immunosupressants, infection and neoplasm is difficult.
In this report, we describe a case of hepatotoxicity by etanercept first and then by adalimumab, in a patient with rheumatoid arthritis who lastly was safely treated with infliximab.
Case report
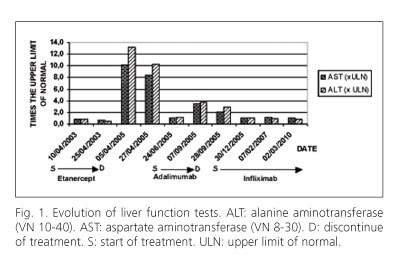
A 47-year-old female diagnosed with rheumatoid arthritis, was attended in an outpatient clinic due to abnormal liver parameters. In April 2003, she received etanercept 25 mg subcutaneous injections twice weekly in addition to previous therapy with methotrexate, deflazacort, meloxicam and folic acid. At the time of starting therapy with etanercept serum aminotransferase levels were within normal range. Two years later she remained well, but ALT was 13.2 times the upper limit of normal (xULN), AST 10.1 xULN and FA 1.7 xULN (Fig. 1). Physical examination was unremarkable. Viral antigens and antibodies for hepatitis A, C, B and cytomegalovirus were all negative. A thorough investigation did not identify any other readily cause of clinically significant liver injury. Etanercept was withdrawn and laboratory findings normalized within 80 days. However, her liver test results were raised again after she was put on adalimumab 40 mg subcutaneous injections once fortnightly for two months (ALT 3.7 xULN, AST 3.5 xULN and FA 1 xULN). Subsequently adalimumab was withdrawn and the patient developed positive titres of antinuclear antibodies (1/320) and two months later liver tests were normal. Then, anakinra (100 mg subcutaneous injection every day) and infliximab (300 mg intravenous every month) treatment were administered. Currently, the patient is being treated with infliximab without recurrence of hepatitis despite continued positive ANA two years later.
Discussion
Liver injury classified by the criteria of the International Consensus Conference (2), corresponded to a hepatocellular pattern. Causality assessment by the use of CIOMS (Council for International Organizations of Medical Science) scale (3), assessed as probable (score of 6) that both etanercept and adalimumab in our case were responsible for the liver damage.
To the best of our knowledge, this is the first report of sequential etanercept and adalimumab associated hepatocelular injury.
TNF-α antagonists induces cytokine imbalance that clinically can be expressed as an infection, neoplastic and or autoimmune conditions, such as lupus, vasculitis, psoriasis, sarcoidosis, seroconvertion of immunologic biomarkers such as ANA, anti-nucleosomal, anti-cardiolipin, and anti-histone antibodies (4).
Most cases of liver toxicity in patients on TNF-α antagonists therapy have been reported with infliximab (1). The US Food and Drug Administration (FDA) has received several spontaneous reports of liver failure during treatment with infliximab and etanercept. Some of these cases were fairly well documented although, other causes of liver disease were present (1). A case report on hetatotoxicity associated with etanercept therapy has been described by Leak and Rincon-Aznar, in a patient with psoriatic arthritis (5). Abnormal liver function tests commenced after the fourth dose of etanercept and normalized after 2 months. In our case, the liver injury occurred after two years and returned to normal at 80 days of etanercept discontinuation. The hepatotoxic potential of adalimumab seems low, with isolated cases published (6). There is a reported case of a patient with psoriatic arthritis who had liver dysfunction during treatment with adalimumab (7).
Previous published cases suggest absence of hepatic cross-toxicity between infliximab and etanercept (8,9), and between adalimumab and etanercept (7). This has been explained because these drugs are structurally different: etanercept is a soluble TNF-receptor fusion protein, and infliximab and adalimumab are chimeric IgG1 anti-TNF-α monoclonal antibodies and by the fact that polymorphisms have been identified in genes encoding proteins related to TNF-α (1,6). However, in our case, the patient experienced liver toxicity with etanercept and adalimumab and did not do with infliximab.
In our patient, high titres of ANA were detected after adalimumab therapy and remained detectable upon resolution of the liver damage. Whether this finding represents unmasking autoimmune hepatitis (AIH) by the drug versus AIH like drug-induced liver injury (AIH-DILI) is unclear. AIH-DILI is a particular phenotype of hepatotoxicity in which elevated titres of autoantibodies (antinuclear antibodies and/or anti-smooth muscle antibodies) become detectable during the liver damage and the bout may fully mimic true AIH. Sometimes patients with AIH-DILI received steroids but discontinuation of immunosuppression once liver tests normalize is typically followed by a durable remission (10). This would be an important clue to distinguish AIH-DILI from true AIH in which clinical and biochemical rebound is quite common after withdrawal of immunosupression (10). Eleven cases of AIH due to anti-TNF-α have been reported between 2001 and 2010 (ten by infliximab and one by adalimumab), and two cases of induction of ANA and the elevation of serum immunoglobulin G levels by infliximab and etanercept (11).
In conclusion, this case report illustrates the complexity of liver injury induced by biological agent from the same class (such as the TNF-α antagonists). If liver injury developed with a TNF-α antagonist switching to another with close liver tests monitoring may be a judicious alternative.
José Carlos Titos-Arcos1, Hacibe Hallal2, Mercedes Robles3,4 and Raúl J. Andrade3,4
1Department of Pharmacy. Hospital General Universitario Morales Meseguer. Murcia, Spain
2Department of Gastroenterology. Hospital General Universitario Morales Meseguer. Murcia, Spain
3Liver Unit. Department of Digestive Diseases. Hospital Universitario Virgen de la Victoria. Málaga, Spain
4Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd). Barcelona, Spain
References
1. Dominique L. Liver toxicity of TNF-alpha antagonists. Joint Bone Spine 2008;75:636-8. [ Links ]
2. Bénichou C. Report of an International Consensus Meeting. Criteria of drug-induced liver disorders. J Hepatol 1990;11:272-6. [ Links ]
3. Danan G., Bénichou C. Causality assessment of adverse reactions to drugs I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323-30. [ Links ]
4. Cuchacovich R, Hagan J, Khan T, Richert A, Espinoza LR. Tumor necrosis factor-alpha (TNF-alpha)-blockade-induced hepatic sarcoidosis in psoriatic artritis (PsA): case report and review of the literature. Clin Rheumatol 2011;30:133-7. [ Links ]
5. Leak AM, Rincon-Aznar B. Hepatotoxicity associated with etanercept in psoriatic arthritis. J Rheumatol 2008;35:2286-7. [ Links ]
6. Toscano E, Cotta J, Robles M, Lucena MI, Andrade RJ. Toxicidad inducida por los nuevos fármacos inmunosupresores. Gastoenterol Hepatol 2010;33:54-65. [ Links ]
7. Massarotti M, Marasini B. Successful treatment with etanercept of a patient with psoriatic arthritis after adalimumab-related hepatotoxicity. Int J Immunopathol Pharmacol 2009;22:547-9. [ Links ]
8. García-Aparicio AM, Rey-Rey J, Hernández-Sanz A, Sampedro J. Successful treatment with etanercept in a patient with hepatotoxicicy closely related to infliximab. Clin Rheumatol 2007;26:811-3. [ Links ]
9. Thiefin G, Morelet A, Heurgue A, Diebold MD, Eschrd JP. Infliximab-induced hepatitis: Absence of cross-toxicity with etanercept. Joint Bone Espine 2008;75:737-9. [ Links ]
10. Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 2010;51:2040-8. [ Links ]
11. Efe C, Purnak T, Ozaslan E, Wahlin S. Drug-induced autoimmune hepatitis caused by anti-tumor necrotis factor agents. Hepatology 2010;52(6):2246-7. [ Links ]











 texto en
texto en