My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.104 n.9 Madrid Sep. 2012
https://dx.doi.org/10.4321/S1130-01082012000900009
Evaluation of the oncogenic risk of diffuse gastric polyposis. A case report
Evaluación del riesgo oncogénico de la poliposis gástrica difusa. Un caso clínico
Erasmo Spaziani1, Marcello Picchio2, Annalisa Di Filippo1, Piero Narilli3, Luca Pacini4, Valentina Moretti4, Pierino Lucarelli5, Francesco De Angelis1, Giuseppe Ragona4 and Vincenzo Petrozza4
1Department of Medico-Surgical Sciences and Biotechnologies. University of Rome Sapienza. Hospital "A. Fiorini". Terracina, Latina, Italy
2Department of Surgery. Hospital "P. Colombo". Velletri, Rome, Italy
3Department of Surgery. General Hospital "Nuova Itor". Rome, Italy
4Department of Medico-Surgical Sciences and Biotechnologies, University of Rome, Sapienza, Latina, Italy
5Department of Surgery. Hospital "Madonna delle Grazie". Velletri, Rome, Italy
Copyright notice: With kind permission from Springer Science + Business Media: Spaziani E, Picchio M, Di Filippo A, Narilli P, Di Cristofano C, Petrozza V, De Angelis F, Ragona G. Sporadic diffuse gastric polyposis: report of a case. Surgery Today 2011; 41(10):1428-31.
ABSTRACT
Benign polyps of the stomach undergo malignant transformation at a rate correlating to the histological type and size of the proliferative lesion. We report a case of a 50-year-old Caucasian woman, affected by a diffuse gastric polyposis of both hyperplastic and adenomatous type. At endoscopy polyps were more than 1,000, scattered over the entire gastric cavity. The patient underwent total gastrectomy. The perilesional gastric mucosa was characterized by the presence of either atrophic or metaplastic areas and by a mild dysplasia. A single tubulo-villous adenomatous polyp was also present in the ascending tract of the colon. The absence of both high-grade dysplastic lesions and outbreaks of neoplastic transformation well correlated with the histochemical and molecular features, confirming the highly proliferative pattern of the polyps in the lack of signs of malignant progression.
Key words: Diffuse gastric polyposis. Hyperplastic polyps. Adenomatous polyps. Malignant potential. Immunohistochemistry. Molecular assay.
Introduction
Benign polyps of the stomach present as a heterogeneous group of lesions. The two most common types are the hyperplastic and the adenomatous polyps, which have distinct features and different rates of progression toward malignancy (1). The rate of malignant transformation is related to the histological type and size of the polyp and it is estimated to be 18% for adenomatous polyps compared to the rate of 1.5-4.5% for hyperplastic polyps, according to recent studies (2,3). Metaplastic changes occur in 16.7% of hyperplastic polyps and in 72.7% of adenomatous polyps, mostly within the perilesional mucosa, which is constantly infiltrated with polymorphonuclear elements (2). Polyps with mixed characteristics, i.e. showing both adenomatous and hyperplastic areas have been also described (4). Patients with adenomatous-hyperplastic polyps feature high levels of gastrin and only occasionally showed to carry Helicobacter pylori infection (5,6). Gastrin is believed to favor neoplastic growth (5,6). In diffuse gastric polyposis, a less common disorder, the gastric mucosa is largely filled with polyps (at least more than 50) and patients are usually affected by severe anemia (5,7).
Among the various pathogenetic hypotheses explaining the histomorphologic progression of gastric lesions, the one proposed by Correa (with the sequence: gastritis-intestinal metaplasia-dysplasia-gastric carcinoma) is greatly favored, whereas the direct sequence from adenoma to carcinoma, though generally accepted for colon cancer, is questioned (8-10).
We report a rare case of diffuse gastric polyposis of both hyperplastic and adenomatous type. Histological and molecular findings of the lesions are discussed with respect to their proliferative pattern and malignant potential.
Case report
Clinical history
A 50-year-old Caucasian woman with severe sideropenic anemia (hemoglobin 4.1 g/dL) had a diagnosis of diffuse gastric polyposis following esophagogastroduodenoscopy. She did not report family history for gastric tumors or familial polyposis of the colon, nor was she ever treated with proton pump inhibitors. Gastrin levels were within the normal range. Seriate radiographs of the small intestine did not show the presence of further polyps. Only a single pedunculated polyp, about 3 cm in diameter, was found in the ascending colon at colonoscopy. It was removed with diathermy. At histological examination, the polyp was tubulo-villous with moderate dysplasia. The patient underwent total gastrectomy and omentectomy. The postoperative course was uneventful. At a follow-up of 3-years after the surgical intervention the patient was found to be in good health.
Pathological findings
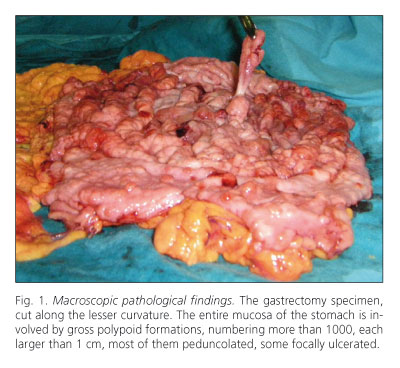
Macroscopically, gastric mucosa appeared to be diffusely covered with gross polypoid formations, numbering more than 1,000, each larger than 1 cm. Most of them were pedunculated, and some were also focally ulcerated (Fig. 1).
Microscopically (Fig. 2A), the majority of polyps were similar in shape and presented with surface glandular formations of hyperplastic morphology, spread over an abundant, fairly edematous layer of fibrotic stroma. Foveolar cells appeared elongated and branched. The luminal profile was irregular, jagged in some portions or dilated for the presence of engulfed cysts. The glandular epithelium was paved with tall cells having a small nucleus, confined toward the basal edge, and abundant eosinophilic cytoplasm. Isolated glands with similar morphology, mostly dilated, were scattered throughout the mucosa. In a few polyps foveolar cells were clustered as a "gland in a gland", and were lined by cells with altered nucleus/cytoplasm ratio, hyperchromatic and elongated nuclei, and with a limited pattern of migration. Within the stroma, a mild inflammatory lympho-plasmacytoid infiltrate and numerous vessels, some of them congested, were present. Based on the overall picture we concluded that the patient was affected by multiple gastric polyposis, with the majority of polyps being hyperplastic, or of adenomatous tubular type, in a few cases with features of serrated adenoma, and characterized by focal glandular low grade dysplasia. Notably, a careful examination of serial sections, taken from multiple areas of the gastric cavity, did not show the presence of areas of in situ malignancy. Atrophic gastritis with intestinal metaplasia was also present, whereas morphological and histochemical investigations for Helicobacter pylori infection were all negative.
Immunohistochemical findings
In order to further characterize the phenotype of the polypoid lesions and the low grade dysplastic components, we investigated by immunohistochemistry the reactivity to antibodies specific for E-cadherin (36B5), beta-catenin (17C2), P53 protein (DO-7), ki-67 nuclear component (MM1) and the EGF receptor (PharmaDX, DAKO, Italy). Except for the EGF receptor, all antibodies were purchased from Novocastra Ltd., UK. Immunohistochemistry was performed with the BondTM automated staining system (Menarini, Italy). Both beta-catenin and E-cadherin were expressed as a membrane diffuse positivity in the glandular epithelium of the polyps and the low grade dysplastic components. P53 protein expression was detected only in the hyperplastic areas with low grade dysplasia (Fig. 2B), the same areas where Ki-67 antigen positive cells were more frequently found. EGF receptor overexpression was detected at the level of the resting cells of the deep portion of foveola. P53 immunoreactivity was defined as positive when a distinct nuclear staining was recognized in at least 10% of the cells. The proliferative index was highest among the dysplastic foci.
Molecular assays
DNA was extracted from either formalin-fixed paraffin-embedded or fresh polypoid tissue and the presence of c-Ki-Ras mutations was investigated with the polymerase chain reaction and direct sequencing of the amplimers. HER2 amplification was analyzed by FISH (PathVysion™, Vysis®, USA).
Sequencing of the amplimers covering the sequence of exons 1 to 5 of c-Ki-Ras gene failed to show the presence of mutations. Similarly, HER2 amplification was not detected.
Discussion
In the case we report the diagnosis of diffuse gastric polyposis was based on the endoscopic examination of the upper digestive tract, which showed the massive involvement of the stomach. The histologic analysis of polypoid lesions reported the proliferation of hyperplastic, adenomatous and hyperplastic-adenomatous mixed polyps with low-grade glandular dysplasia, thus confirming the findings of a previous large series (4).
The polypoid proliferation was associated with chronic atrophic gastritis in the perilesional mucosa and intestinal metaplasia, whereas Helicobacter pylori infection, high-grade glandular dysplasia or invasive adenocarcinoma were all absent. Our case confirms that the association of Helicobacter pylori infection with diffuse hyperplastic-adenomatous gastric polyposis may not be a frequent event (6).
It has been shown that gastric carcinogenesis is a multi-step process, supported by multiple genetic and molecular alterations, and mutations at both the c-Ki-Ras gene and the HER2 gene are involved (10-12). We speculate that the evolutive potential of precancerous gastric lesions into frank neoplasia may be assumed by immunohistochemistry and molecular assay.
Point mutations in the genes of the E-cadherin-catenin functional complex can be responsible for a reduced level of expression on the plasma membrane, thus decreasing cell to cell adhesiveness and increasing invasiveness and metastatic potential both in the early and late stages of carcinogenesis (13). Overexpression of p53 protein in gastric hyperplastic and adenomatous polyps is also taken as an indicative marker of progressive dysplasia (3). In addition, the frequency of cells immunoreactive to Ki-67 antibody, a cell proliferation marker commonly found in the hyperproliferative foveolar areas of the gastric mucosa, also increases with the rate of dysplasia (3,9). The immunohistochemical analysis has shown in this case that both beta-catenin and the cell-to-cell adhesion molecule E-cadherin were normally expressed in the normal glandular epithelium as well as in the dysplastic areas. Therefore, our data seem to exclude the onset of mutations in these genes at the time of diagnosis. The increased expression of Ki-67 in the hyperplastic and dysplastic components confirms the frequent tendency of polypoid lesions to progress toward enhanced proliferation. This finding is substantiated also by the p53 nuclear staining in the hyperplastic areas with low grade dysplasia. Our data are in agreement with previously published observations stating that overexpression of p53 protein can be recognized in both the dysplastic and carcinomatous components of gastric polyps (3,9,14). P53 accumulation in dysplastic areas of gastric polyps may favour early carcinogenetic events (3). Therefore, in this setting, even low grade dysplasia might have the potential of developing into carcinoma, based on the high proliferative activity of the lesions and their high positivity for p53 protein. On the other hand the overexpression of EGFR, the membrane receptor that binds several growth factors, regulating cell differentiation and proliferation in gastric mucosa, has been localized by immunohistochemistry to the resting cells of the deep portion of foveola, where the mitogenic stimulus seems to be restricted (15). As well the absence of point mutations in the c-Ki-ras gene and the lack of HER2 amplifications, in our case should not be surprising because of the absence of frankly neoplastic areas.
Finally, the association in the present case of diffuse hyperplastic-adenomatous gastric polyposis and the solitary colorectal adenomatous polyp might not be fortuitous. Further studies are underway to find a possible genetic correlation.
In conclusion, we described a case of diffuse gastric polyposis which at histopathological examination showed the absence of both high-grade dysplastic lesions and outbreaks of neoplastic transformation. These findings correlated well with either the results of the histochemical and molecular investigations, confirming the highly proliferative pattern of the polyps in the lack of signs of malignant progression.
References
1. Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985;20(Pt 1):303-29. [ Links ]
2. Espejo Romero LH, Navarrete Siancas J. Gastric epithelial polyps. Rev Gastroenterol Peru 2003;23:277-92. [ Links ]
3. Yao T, Kajiwara M, Kuroiwa S, Iwashita A, Oya M, Kabashima A, et al. Malignant transformation of gastric hyperplastic polyps: alteration of phenotypes, proliferative activity, and p53 expression. Hum Pathol 2002;33:1016-22. [ Links ]
4. Roseau G, Ducreux M, Molas G, Ponsot P, Amouyal P, Palazzo L, et al. Epithelial gastric polyps in a series of 13000 gastroscopies. Presse Med 1990;19:650-4. [ Links ]
5. Niv Y, Delpre G, Sperber AD, Sandbank J, Zirkin H. Hyperplastic gastric polyposis, hypergastrinaemia and colorectal neoplasia: a description of four cases. Eur J Gastroenterol Hepatol 2003;15:1361-6. [ Links ]
6. Varis O, Laxèn F, Valle J. Helicobacter pylori infection and fasting serum gastrin levels in a series of endoscopically diagnosed gastric polyps. APMIS 1994;102:759-64. [ Links ]
7. Jayawardena S, Anandacoomaraswamy D, Burzyantseva O, Abdullah M. Isolated diffuse hyperplastic gastric polyposis presenting with severe anemia. Cases J 2008;1:130. [ Links ]
8. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-fisrt American Cancer Society Award Lecture on cancer epidemiology and prevention. Cancer Res 1992;52:6735-40. [ Links ]
9. Sugai T, Inomata M, Uesugi N, Jiao YF, Endoh M, Orii S, et al. Analysis of mucin, p53 protein and Ki-67 expressions in gastric differentiated-type intramucosal neoplastic lesions obtained from endoscopic mucosal resection samples: a proposal for a new classification of intramucosal neoplastic lesions based on nuclear atypia. Pathol Int 2004;54:425-35. [ Links ]
10. Tanaka A, Takemura-Tsukashita S, Kushima R, Sugihara H, Fujiyama Y, Hattori T. Low-grade gastric adenomas/dysplasias: phenotypic expression, DNA ploid pattern, and LOH at microatellites linked to the APC gene. Pathol Res Pract 2008;204:1-9. [ Links ]
11. Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [ Links ]
12. Shen C, Chang JG, Lee LS, Yang MJ, Chen TC, Lin KY, et al. Analysis of ras gene mutations in gastrointestinal cancers. J Formos Med Assoc 1991;90:1149-54. [ Links ]
13. Wijnhoven BPL, Dinjens WNM, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 2000;87: 992-1005. [ Links ]
14. Lauwers GY, Wahl SJ, Melamed J, Rojas-Corona RR. P53 expression in precancerous gastric lesions: an immunohistochemical study of Pab 1801 monoclonal antibody on adenomatous and hyperplastic gastric polyps. Am J Gastroenterol 1993;88:1916-9. [ Links ]
15. Abe S, Sasano H, Katoh K, Ohara S, Arikawa T, Noguchi T, et al. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig Dis Sci 1997;42:1199-209. [ Links ]
![]() Correspondence:
Correspondence:
Annalisa Romina Di Filippo
e mail: annalisa.difilippo@alice.it
Received: 24-02-2012
Accepted: 25-06-2012