My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 n.5 Madrid May. 2014
ORIGINAL PAPERS
Preparations for colon capsule endoscopy. Prospective and randomized comparative study between two preparations for colon capsule endoscopy: PEG 2 liters + ascorbic acid versus PEG 4 liters
Preparaciones para endoscopia del colon con cápsula. Estudio prospectivo, aleatorio y comparativo entre dos preparaciones del colon para cápsula endoscópica: 2 litros PEG + ácido ascórbico versus 4 litros PEG
Federico Argüelles-Arias1, Mileidis San-Juan-Acosta1, Alba Belda1, Josefa María García-Montes1, Francisco Pellicer1, Juan Polo2, Ángel Caunedo-Álvarez1 and Juan Manuel Herrerías-Gutiérrez1
1UGC Digestive Diseases. 2Unit of Statistics. Hospital Universitario Virgen Macarena. Sevilla, Spain
ABSTRACT
Introduction: PillCam© colon capsule endoscopy (CCE) enables the study of colonic diseases in a safe and non-invasive way, although there are aspects that need to be improved. Current methods of bowel preparation lead to discordant rates of adequate cleansing and CCE excretion.
Aims: To compare the efficacy of colon cleansing using two different regimes (2L PEG plus ascorbic acid versus 4L PEG alone) for PillCam Colon (C2) capsule endoscopy.
Methods: Fifty eight patients included in this prospective study and randomized to: Group A, PEG plus ascorbic acid regimen (n = 28, 12 F/16 M) or group B, PEG alone regimen (n = 30, 14 F/16 M). The degree of cleansing was categorized into "excellent-good" or "fair-poor", according to Leighton's recently published preparation scale. CCE excretion rate and colon cleansing were assessed. Patients underwent to PillCam colon of second generation (C2).
Results: Cleansing was considered to be excellent-good in 78 % of cases in group A and in 64 % of cases in group B, with no significant difference between the groups (p = 0.252). Nevertheless, when the grade of cleansing was analyzed in segments, a significant difference was found in the cecum and transverse colon. No differences were observed in the bubble effect between preparations. The excretion rate was 93 % in group A versus 70 % in group B (p = 0.043).
Conclusions: These results suggest that a 2L PEG plus ascorbic acid regimen is at least as effective as a 4L PEG regimen. This regimen may be considered an effective alternative which would improve compliance because a smaller volume is required.
Key words: Colon capsule endoscopy. Bowel preparation. PEG plus ascorbic acid.
Introduction
Colorectal cancer (CRC) is one of the most prevalent causes of morbidity and mortality in western countries (1). Conventional colonoscopy is the gold standard used in cancer screening programmes, but this technique is invasive, and in some cases painful. PillCam© colon capsule endoscopy (CCE; Given Imaging Ltd, Yoneam, Israel) is a new, safe and non-invasive endoscopic tool that may allow exploration of the colon without sedation and gas insufflation, which thus represents a good alternative to conventional endoscopy. Nevertheless, there are some aspects of the technique that could be improved. Recently a new second-generation CCE has been developed that provides a higher number of images per second and a larger viewing angle, which achieves better results in CRC screening (2).
The other important aspect is the preparation of the bowel. CCE cannot insufflate the colon, aspirate liquids, or wash the mucosal surface during the procedure, so bowel cleansing has to be better for CCE than for conventional colonoscopy. Current methods of preparation led to discordant rates of adequate cleansing or CCE excretion. Recently a guideline (3) for the use of CCE has been published which contains some statements and recommendations about bowel preparation.
The colon preparation for CCE has two aims: Firstly it must provide a clean colon and clear images and secondly, it must promote capsule propulsion, initially through the entire small bowel and then through the colon to the rectum. The classic colonoscopy preparation for CCE (4L PEG regimen and NaP booster) is not enough to achieve these two aims and new protocols have therefore been designed. This conventional preparation was first evaluated in two pilot studies. In the Eliakim et al. (4) study, the overall cleanliness of the colon was rated as excellent or good in 84 % of cases. In the second pilot study (5) the results are better; an excellent or good preparation was achieved in 90 % of cases. In the largest study, the Van Gossum et al. study (6), the preparation was excellent or good in 72 % of patients. In a recent study published by our group using the same preparation, the grade of cleanliness was rated as excellent or good in 66 % (7).
It appears, as it has been mentioned, that colon cleanliness significantly influences the sensitivity of capsule endoscopy. The published studies show an overall adequate cleansing level in less than 70 % of patients (approximately). This means that more than 1 in every 4 patients has an inadequate degree of colon cleansing. In addition, 4 liters of PEG is the recommended volume, and it is known that this amount of preparation is not as well tolerated as 2 liters of preparation. A large volume taken over a short period of time may result in patient intolerance and poor compliance, leading to poor colon cleansing.
The aims of our study were therefore:
- To compare the degree of colon cleansing using two different regimes (PEG plus ascorbic acid versus PEG) prior to PillCam Colon (C2) capsule endoscopy.
- To assess the capsule excretion rate using the two different preparations.
- To evaluate the transit time with the preparations.
Material and methods
This was a prospective, randomized trial to study the grade of colon cleansing using two preparations. This study was approved by the appropriate ethics committee for clinical research.
The inclusion criteria were: patients older than 18 years and undergoing colo-rectal cancer screening. The exclusion criteria were the same as those used for the small bowel capsule, the key criteria being difficulty swallowing the capsule and the presence of a stricture in the gastrointestinal tract. In addition, patients at increased risk of sodium phosphate toxicity (8) (patients with hypovolemia, baseline kidney disease, bowel obstruction, or active colitis, patients on medications affecting renal perfusion or with function kidney failure, or receiving concomitant treatment with angiotensin-converting enzyme inhibitors) and/or allergic to any of the medications of the study were excluded.
Written informed consent was obtained from each participant, and the study was approved by the institutional review board of the study site.
Sample size calculation with an α = 0.05 and a power (1-β) = 0.8, was 60 patients, 30 for each arm, based on the Spada's et al. study (9). The nQuery Advisor® program was used to calculate the population in each arm. Patients were randomized, alternate, 1:1, into two groups. The groups were the following:
- Group A: PEG 2 liters plus ascorbic acid.
- Group B: PEG 4 liters.
The preparation schedule is shown in table I.
Colon capsule endoscopy
Patients underwent to PillCam colon of second generation (C2) (Given Imaging Ltd., Yoqneam, Israel). This model measures 11.6 by 31.5 mm with two imagers, one at each end of the capsule. The angle of view has been increased to 172 degrees and to conserve battery energy, the capsule captures images at an adaptive frame rate, alternating from 35 frames per second while it is in motion (such as in the transverse colon) to 4 images per second when it is virtually stationary. After swallowing, the capsule works with a low frame rate of 14 per minute until it automatically identifies the small bowel. The new data recorder also assists and guides the medical staff and patient through the procedure. It buzzes, vibrates and displays instruction numbers in order to alert the patient to take the laxative booster or that the procedure has terminated.
The need for good bowel preparation was emphasized to the patients by an expert nurse in endoscopic procedures, since the quality of bowel preparation has been found to relate to the accuracy of the CCE procedure.
A detailed instruction form was provided to all patients.
Study outcome measures
Colon cleansing levels
The overall degree of cleansing was determined according to Leighton's recently published (10) preparation scale, but divided in two categories "excellent-good" or "fair-poor". Also the grade of cleansing was analyzed in segments (cecum, ascending colon, transverse colon, descending colon and rectum).
The definitions for each category were as follows:
- Excellent: Full visibility of the mucosa in the segment of the colon, with no more than small bits of remaining feces.
- Good: Quite complete examination of the mucosa, despite the presence of small amounts of solid material or dark liquid in the segment.
- Fair: Impaired visibility of the mucosa by solid feces or a large amount of dark fluids.
- Poor: Examination of the mucosa possible for less than 25 % of the circumference.
The scale includes the effect of bubbles in the preparation: significant (more than 10 % of the surface area is obscured by bubbles) or insignificant (less than 10 % of the surface area is obscured by bubbles).
CCE excretion rate and transit time
CCE was considered "complete" when the hemorrhoidal plexus was identified. Transit time to the colon was measured from the RAPID video for each patient and was defined as the time from initial ingestion to the time of first colon image. In addition, transit time within the colon was measured from the RAPID video for each patient.
Two selected clinicians who were blinded to the study groups graded the cleanliness level, the excretion rate and the transit time (FAA and ACA). An additional independent physician supervised the blinding process (JMHG).
Statistical analysis
Descriptive statistics were performed for patient demographics. The chi-squared test and Fisher's exact test were performed to compare between the two cleansing procedures and to investigate whether there were statistically significant differences in the complete excretion of CCE or not. Also the Student's test was used to analyze quantitative variables as transit time of CCE. IBM SPSS Statistics 22.0® program was used.
Results
Sixty patients were included in the study, although finally 58 patients were analyzed. In one patient the CCE was delayed in the esophagus because of an unknown peptic stricture and was retrieved via gastroscopy and in another case the patient was excluded because of preparation intolerance (both in group A).
Baseline characteristics are included in table II. A total of 16 males and 12 females were included in group A, and 16 males and 14 females were included in group B (p = NS). The mean age was 56.5 ± 17.3 years in group A and 54.5 ± 16.05 in group B (p = NS). All of the patients were in a protocol of CRC screening.
Colon cleansing levels
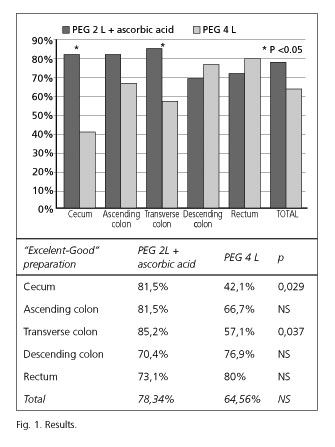
The cleansing grade was considered as excellent-good in 78.34 % of cases in group A and in 64.56 % of cases in group B, with no significant difference between the two groups (p = 0.252). Nevertheless, when the grade of cleansing was analyzed in segments, a statistically significant difference was found in the cecum and transverse colon (Fig. 1). No differences were observed in the bubble effect between the two preparations. In the cecum, the bubble effect was not significant in 85.2 % of patients in group A versus 83.3 % in group B (p = NS). In the ascending colon, the bubble effect was not significant in 88.9 % of patients in group A versus 90 % in group B (p = NS). In the transverse colon, the bubble effect was not significant in 81.5 % of patients in group A versus 75 % in group B (p = NS). In the descending colon, the bubble effect was not significant in 77.8 % of patients in group A versus 76.9 % in group B (p = NS). In the rectum, the bubble effect was not significant in 84.6% of patients in group A versus 84.9 % in group B (p = NS).
CCE excretion and capsule transit time
As shown in table III, the excretion rate was 92.9 % in group A versus 70 % in group B (p = 0.043). In group A the hemorrhoidal plexus was not seen only in one patient but in group B the hemorrhoidal plexus was not seen in 6 patients. Three capsules were delayed in the transverse colon, one in the ascending colon, and two in the descending colon. The mean transit time was 387 ± 184 minutes in group A and 370 ± 144 minutes in group B (p = NS). The mean colon transit time was 160 ± 185 minutes in group A and 146 ± 102 minutes in group B (p = NS).
Discussion
Good preparation for CCE is essential because as Spada et al. (11) have demonstrated, when the preparation is adequate, the accuracy of CCE tends to be higher. In this paper, the quality of colon preparation during CCE was described as adequate in 43.5 % patients, with the remaining 56.5 % patients having inadequate colon preparation. When the level of colon cleansing was adequate, the sensitivity was 91 % and the specificity 97 %. When there was inadequate cleansing however, the sensitivity was only 38 % and the specificity 90 %. These authors thus consider there to be a strong relationship between the quality of cleansing and CEE sensitivity. In the largest study of CCE (4) the sensitivity was significantly higher in patients with good or excellent cleansing compared to those with poor or fair cleansing. The sensitivity and specificity for the detection of polyps (≥ 6 mm) in patients with good or excellent cleansing was 75 % and 84 %, respectively, and for the detection of such polyps in patients with poor or fair cleansing, the sensitivity and specificity was 42 % and 84 %, respectively.
A recent paper (12) described a new regimen of preparation consisting of a split dose of PEG and a 45 mL dose of sodium phosphate (NaP). Four senna tablets and a low-residue diet were also included. CCE excretion rate, colon cleansing, and accuracy were assessed. At CCE, the bowel preparation was rated as good in 78 % of patients, fair in 20 % and poor in 2 %. CCE excretion occurred in 83 % of patients. The authors concluded that the combination of a split-dose of PEG solution alongside a low dose of NaP boosters resulted in high rates of adequate cleansing and CCE excretion. In another recently published (13) study the findings of a single center are reported. For colon cleansing they applied their department's standard preparation procedure for colonoscopy including a low-fiber diet and PEG, and added an oral motility agent, Phospho Soda-boosters and a suppository. The level of cleansing on CCE was good in 15 (27 %), moderate in 30 (54 %) and poor in 11 (20 %) cases. Nevertheless, they found a lower excretion rate for CCE (64 %, n = 36) than the two previous pilot studies. This might have been caused by an additional fasting time of almost 4 hours between ingestion of the second 2 liters of PEG and initiation of CCE. Also, in the recent European Colon capsule endoscopy consensus (2) based on the studies that have been developed, some recommendations have been included. Patients need to follow a liquid diet the day before the procedure and it is necessary to administer 4 liters of a PEG solution with a split regimen, to increase the tolerability and the efficacy of the preparation as has been demonstrated recently (11). The administration of boosters during the evolution of the capsule is necessary for the progression of the capsule, and also allows the capsule to move in a watery environment. In this sense, a low dose of NaP (45 mL) has been shown to achieve an adequate CCE excretion (14).
The efficacy of bowel cleansing for conventional colonoscopy with 4 L PEG regimens varies widely, with 33 % to 83 % of patients achieving good or excellent cleansing (15-22). It is well known that reduced-volume PEG products generally provide comparable colon cleansing compared with 4 L PEG preparations (23,24). Thus, we conducted this study to assess whether 2 L PEG + ascorbic acid preparations could be as useful as 4 L PEG preparations in preparing the colon for CCE. Ascorbic acid is mainly absorbed in the small intestine by an active transport mechanism, sodium-dependent and saturable. These results in an inverse relationship between the doses ingested and absorbed dose rate. The unabsorbed fraction of ascorbic acid appears to act as an osmotic agent in the intestinal lumen leading to osmotic evacuation. Senna is used to stimulate colonic peristalsis and as the primary cleansing agent with a liquid diet. With that intention four senna tablets and a low-residue diet were included in our schedule.
The cleansing grade was considered as excellent-good in 78.34 % of cases in the group prepared with 2 L PEG and in 64.56 % of cases in the group prepared with 4 L PEG. Although no statistically significant difference was observed in the overall preparation, we observed significantly better cleansing in the cecum and transverse colon using 2 L PEG plus ascorbic acid. Perhaps, the number of patients included in the study is small to consider that difference as statistically significant. Nevertheless, we calculated the two groups based in a recent published paper (9). Without any doubt, a bigger study should be performed. As some studies have demonstrated (25) that low volume PEG plus ascorbic acid is more acceptable to patients, the preparation may be even better in this group than in the 4 L PEG one. Recently, Hartmann et al. (26) published a study using PEG plus ascorbic acid in CCE preparation using PEG as a booster. The cleansing level was > 80 %, approximately the same as our result, and they concluded that this regimen would be used in patients in whom sodium phosphate-based preparations are used.
As mentioned above, the published studies showed an overall adequate cleansing level of between 35-90 % (4-7,9,12) so the ideal preparation has not yet been found. To improve the tolerability and patient compliance a reduced volume preparation could be used, because the cleansing level is acceptable in our study. The excretion rate is also better in group A than in group B (92.9 % vs. 70 %). This excretion rate is far higher than the rate in the published studies, which varies between 69 and 84 %) (4-7,9,12). We have no reason for this, and perhaps new studies should be performed to investigate it. In the Hartmann study (24) using PEG as a booster, the completion rate was 76 %, similar to the rates published.
In conclusion, our study shows that a low volume PEG plus ascorbic acid preparation is at least as good as 4 L PEG, and even better in some segments, with an enhanced excretion rate. We therefore propose that this low volume preparation plus NAP booster is used for CCE as the standard preparation.
References
1. Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92. [ Links ]
2. Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009;41:1026-31. [ Links ]
3. Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2012;44:527-36. [ Links ]
4. Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: Results of the first multicenter, prospective, comparative study. Endoscopy 2006;38:963-70. [ Links ]
5. Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: A prospective pilot study. Endoscopy 2006;38:971-7. [ Links ]
6. Van Gossum A, Navas MM, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009;361:264-70. [ Links ]
7. Herrerías-Gutiérrez JM, Argüelles-Arias F, Caunedo-Álvarez A, San-Juan-Acosta M, Romero-Vázquez J, García-Montes JM, et al. PillCamColon Capsule for the study of colonic pathology in clinical practice. Study of agreement with colonoscopy. Rev Esp Enferm Dig 2011;103:69-75. [ Links ]
8. US Food and Drugs Administration. Oral sodium phosphate (OSP) products for bowel cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). Available at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm103354.htm (Accessed: 11 March 2011). [ Links ]
9. Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, Costamagna G. PillCam colon capsule endoscopy: A prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol 2011;45:119-24. [ Links ]
10. Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: A reliability study. Endoscopy 2011;43:123-7. [ Links ]
11. Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, De Vincentis F, et al. PillCam Colon Capsule Endoscopy (PCCE): The Quality of Preparation Makes the Difference! Gastrointest Endosc 2010;5:AB203-4. [ Links ]
12. Spada C, Hassan C, Ingrosso M, Repici A, Riccioni ME, Pennazio M, et al. A new regimen of bowel preparation for PillCam colon capsule endoscopy: A pilot study. Dig Liver Dis 2011;43:300-4. [ Links ]
13. Pilz JB, Portmann S, Peter S, Beglinger C, Degen L. Colon capsule endoscopy compared to conventional colonoscopy under routine screening conditions. BMC Gastroenterol 2010;10:66. [ Links ]
14. Sieg A, Friedrich K, Sieg U. Is PillCam COLON capsule endoscopy ready for colorectal cancer screening? A prospective feasibility study in a community gastroenterology practice. Am J Gastroenterol 2009;104:848-54. [ Links ]
15. Allaire J, Thompson WO, Cash BD, Galt DJ. A quality improvement project comparing two regimens of medication for colonoscopy preparation. Gastroenterol Nurs 2004;27:3-8. [ Links ]
16. Cohen SM, Wexner SD, Binderow SR, Nogueras JJ, Daniel N, Ehrenpreis ED, et al. Prospective, randomized, endoscopic-blinded trial comparing precolonoscopy bowel cleansing methods. Dis Colon Rectum 1994;37:689-96. [ Links ]
17. Huppertz-Hauss G, Bretthauer M, Sauar J, Paulsen J, Kjellevold Ø, Majak B, et al. Polyethylene glycol versus sodium phosphate in bowel cleansing for colonoscopy: A randomized trial. Endoscopy 2005;37:537-41. [ Links ]
18. Hwang K-L, Chen WT-L, Hsiao K-H, Chen HC, Huang TM, Chiu CM, et al. Prospective randomized comparison of oral sodium phosphate and polyethylene glycol lavage for colonoscopy preparation. World J Gastroenterol 2005;11:7486-93. [ Links ]
19. Kolts BE, Lyles WE, Achem SR, Burton L, Geller AJ, MacMath T. A comparison of the effectiveness and patient tolerance of oral sodium phosphate, castor oil, and standard electrolyte lavage for colonoscopy or sigmoidoscopy preparation. Am J Gastroenterol 1993;88:1218-23. [ Links ]
20. Oliveira L, Wexner SD, Daniel N, DeMarta D, Weiss EG, Nogueras JJ, et al. Mechanical bowel preparation for elective colorectal surgery. A prospective, randomized, surgeon blinded trial comparing sodium phosphate and polyethylene glycol based oral lavage solutions. Dis Colon Rectum 1997;40:585-91. [ Links ]
21. Seinela L, Pehkonen E, Laasanen T, Ahvenainen J. Bowel preparation for colonoscopy in very old patients: a randomized prospective trial comparing oral sodium phosphate and polyethylene glycol electrolyte lavage solution. Scand J Gastroenterol 2003;38:216-20. [ Links ]
22. Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol 1990;85:422-7. [ Links ]
23. DiPalma JA, Wolff BG, Meagher A, Cleveland Mv. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003;98:2187-91. [ Links ]
24. Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006;72:909-11. [ Links ]
25. Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Rünzi M, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008;103:883-93. [ Links ]
26. Hartmann D, Keuchel M, Philipper M, Gralnek IM, Jakobs R, Hagenmüller F, et al. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy 2012;44:482-6. [ Links ]
![]() Correspondence:
Correspondence:
Federico Argüelles-Arias.
UGC Digestive Diseases.
Hospital Universitario Virgen Macarena.
Avda Dr. Fedriani s/n.
41071 Sevilla, Spain
e-mail: farguelles@telefonica.net
Received: 12-12-2013
Accepted: 18-06-2014