Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 no.5 Madrid may. 2014
REVIEW
Innate lymphoid cells and natural killer T cells in the gastrointestinal tract immune system
Células linfoides innatas y células T natural killer en el sistema inmune del tracto gastrointestinal
Enrique Montalvillo1, José Antonio Garrote1,2, David Bernardo3 and Eduardo Arranz1
1Laboratorio de Inmunología de las Mucosas. IBGM, Universidad de Valladolid-CSIC. Valladolid, Spain.
2Department of Clinical Analysis. Hospital Universitario Río Hortega. Valladolid, Spain.
3Antigen Presentation Research Group. Imperial College London, Northwick Park & St Mark's Campus. Harrow, United Kingdom
This research was supported by Instituto de Salud Carlos III-Fondos FEDER (PI10/01647) (to EA); Junta de Castilla y León (SAN673/VA22-08) (to JAG), "BBSRC Institute Strategic Programme for Gut Health and Food Safety BB/J004529/1", United Kingdom (to DB), and Beca FPI-Junta de Castilla y León/Fondo Social Europeo (to EM).
ABSTRACT
The gastrointestinal tract is equipped with a highly specialized intrinsic immune system. However, the intestine is exposed to a high antigenic burden that requires a fast, nonspecific response -so-called innate immunity- to maintain homeostasis and protect the body from incoming pathogens. In the last decade multiple studies helped to unravel the particular developmental requirements and specific functions of the cells that play a role in innate immunity. In this review we shall focus on innate lymphoid cells, a newly discovered, heterogeneous set of cells that derive from an Id2-dependent lymphoid progenitor cell population. These cells have been categorized on the basis of the pattern of cytokines that they secrete, and the transcription factors that regulate their development and functions. Innate lymphoid cells play a role in the early response to pathogens, the anatomical contention of the commensal flora, and the maintenance of epithelial integrity. Amongst the various innate lymphoid cells we shall lay emphasis on a subpopulation with several peculiarities, namely that of natural killer T cells, a subset of T lymphocytes that express both T-cell and NK-cell receptors. The most numerous fraction of the NKT population are the so-called invariant NKT or iNKT cells. These iNKT cells have an invariant TCR and recognize the glycolipidic structures presented by the CD1d molecule, a homolog of class-I MHC molecules. Following activation they rapidly acquire cytotoxic activity and secrete both Th1 and Th2 cytokines, including IL-17. While their specific role is not yet established, iNKT cells take part in a great variety of intestinal immune responses ranging from oral tolerance to involvement in a number of gastrointestinal conditions.
Key words: Intestinal innate immunity. Innate lymphoid cells. Natural killer T cells. Invariant NKT cells. CD1d. Inflammatory bowel disease.
RESUMEN
El tracto gastrointestinal está equipado con un sistema inmune intrínseco altamente especializado. Sin embargo, el intestino soporta una gran carga antigénica que requiere de una respuesta rápida e inespecífica, denominada inmunidad innata, para mantener la homeostasis y proteger al organismo de la entrada de patógenos. En la última década, numerosos estudios han contribuido a desentrañar los requisitos particulares de desarrollo y las funciones específicas de las células que intervienen en la inmunidad innata. En esta revisión, nos centraremos en las células linfoides innatas, un nuevo y heterogéneo grupo de células derivadas de una población linfoide progenitora Id2-dependiente. Estas células han sido categorizadas en base al patrón de citocinas que producen y los factores de transcripción que regulan su desarrollo y funciones. Las células linfoides innatas intervienen en la respuesta temprana contra agentes patógenos, la contención anatómica de la flora comensal, y el mantenimiento de la integridad epitelial. Dentro de las distintas células linfoides innatas haremos especial hincapié en una subpoblación con diversas particularidades, las células T natural killer, un subtipo de linfocitos T que expresan receptores de células T y NK. La fracción más numerosa de células NKT son las denominadas NKT invariantes o iNKT. Las células iNKT, poseen un TCR invariante y reconocen estructuras glicolípidicas presentadas por la molécula CD1d, homóloga de MHC de clase I. Tras su activación, adquieren rápidamente actividad citotóxica y producen citocinas tanto Th1 como Th2, e incluso IL-17. Aunque su papel concreto no está determinado, las células iNKT intervienen en una gran variedad de respuestas inmunes intestinales, desde la tolerancia oral hasta su implicación en diversas patologías del tracto gastrointestinal.
Palabra clave: Inmunidad innata intestinal. Células linfoides innatas. Células T natural killer. Células NKT invariantes. CD1d. Enfermedades inflamatorias intestinales.
Abbreviations list:
IEC, intestinal epithelial cell;
LP, lamina propria;
IELs, intraepithelial lymphocytes;
DC, dendritic cell;
ILC, innate lymphoid cell;
NKT, natural killer T cell;
PRR, pattern recognition receptor;
APC, antigen-presenting cell;
NK, natural killer;
LTi, lymphoid tissue inducer;
IL, interleukin;
IFN, interferon;
IBD, inflammatory bowel disease;
NCR, natural cytotoxicity receptor;
TNF, tumor necrosis factor;
CD, Crohn's disease;
MHC, major histocompatibility complex;
TCR, T-cell receptor;
iNKT, invariant NKT cell;
mNKT, mucosal NKT cell;
vNKT, variant NKT cell;
xNKT, NKT-like cell;
aGalCer, a-galactosylceramide;
iGb3, isoglobo-trihexosylceramide;
Treg, regulatory T cell;
CoD, coeliac disease;
UC, ulcerative colitis.
Gastrointestinal tract immune system
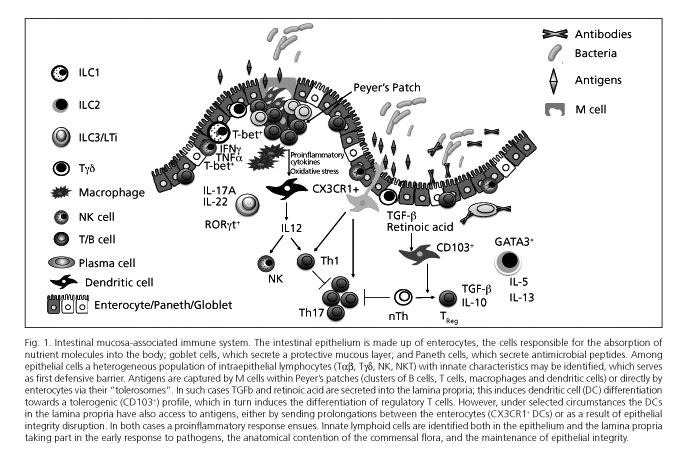
The gastrointestinal tract possesses the highest concentration of immune cells in the human body, and is continually exposed to a high antigenic burden made up not only of nutrients but also the saprophytic intestinal flora (1). The intestinal mucosa includes a first defensive barrier of intestinal epithelial cells (IECs), or enterocytes, that maintain epithelial integrity and are also specialized in the absorption of fluids and nutrients. In addition, the mucosa is equipped with a highly specialized intrinsic immune system (Fig. 1) that ensures optimal nutrient transportation and prevents translocation of bacteria, whether commensal or pathogenic. The intestinal mucosa-associated lymphoid tissue includes lymphoid-cell aggregates such as Peyer's patches and small-bowel mesenteric lymph nodes, as well as isolated lymphoid follicles in the large bowel, which are involved in antigen uptake, processing, and presentation. It also includes a great variety of lymphoid cells in the lamina propria (LP), and intraepithelial effector lymphocytes (IELs) interspersed in the epithelial lining (2).
Both innate and adaptive immune responses represent an integral system of host defense. Their major difference is the specificity of the adaptive immune response, which improves with successive contacts with an antigen but also requires a longer development period. However, the intestine is continually undergoing high antigenic burdens requiring a fast though nonspecific response to maintain intestinal homeostasis and protect the body against incoming pathogens. This response is dependent upon innate immunity (3).
In the past decade numerous reports have unraveled the particular developmental needs and specific functions of cells playing a role in intestinal innate immunity, including monocytes/macrophages, eosinophils, dendritic cells (DCs), and a heterogeneous, newly identified set of innate lymphoid cells (ILCs). In this review we shall focus on the various ILC types in the gastrointestinal tract, and their contribution to intestinal immunity both in health and disease, with an emphasis on a special innate lymphoid subset, namely natural killer T-cells or NKTs.
Innate non-lymphoid cells
While the integrity of junctions between epithelial cells and the differentiation of some of these cells into mucus-secreting goblet cells are vitally important for the defense of the intestinal mucosa, the fact that enterocytes play a much more complex role in the immune response than merely by regulating permeability is now increasingly clear (4). Similar to classic immune cells, IECs also express several pattern recognition receptors (PRRs), which allows them to identify the appropriate ligands produced both by commensal agents and intestinal pathogens. The relevance of these PRRs in IECs may be seen, for instance, in Paneth cells, a subtype of IECs with the ability to detect signals from the commensal flora and to respond by secreting various antimicrobial peptides, thus contributing to maintain intestinal homeostasis. In view of their ability to regulate the saprophytic intestinal flora, some authors have recently suggested that IECs may be considered innate cells on their own right (5).
DCs are the most powerful antigen presenting cells (APCs) in the body, and serve as immune system centinels by their expression of numerous PRRs, including toll-like receptors (TLRs) (6,7). DCs may also activate in the presence of innate signals such as cytokines and/or reactive oxygen species (8). Therefore, DCs represent a nexus between innate (non-antigen-specific) immune response and the highly specialized adaptive immune response. The role of intestinal DCs has been recently reviewed in this journal (9). Under normal conditions, epithelial cells favor immature DC differentiation to tolerogenic DCs thanks to their TGFβ production and retinoic acid synthesis from vitamin A. These tolerogenic DCs controlling oral tolerance mechanisms are CD103+. However, other subtypes of intestinal DCs exist, including the CX3CR1+ population, that can send prolongations between epithelial cells and reach the intestinal lumen to directly take up antigens there. These CX3CR1+ DCs are capable of inducing Th17 responses in vitro (10), and of destroying certain bacteria (11-13); however, they cannot migrate to mesenteric lymph nodes (14). From all the above, CX3CR1+ DCs more closely resemble macrophages than classic DCs (4,15). Such observations have kindled interest in intestinal macrophages, which are the most abundant mononuclear cells in the bowel, play a role in antigen presentation within the lamina propria, and are key in maintaining intestinal homeostasis (11,16).
Until now eosinophils were considered to be IgE-dependent effector cells in inflammatory processes such as allergic hypersensitivity and parasite infestation. However, under normal conditions, eosinophils are commonly seen in the intestinal mucosa and authors such as Svenson-Frej et al. (17) advocate the notion that these cells play a crucial role in intestinal homeostasis. Other authors, as Johnsson et al. (18), note more conventional properties in these intestinal cells, and suggest their active role in various diseases, including ulcerative colitis, eosinophilic esophagitis, and respiratory allergies.
In summary, non-lymphoid cells with innate characteristics are of vital importance in gastrointestinal tract defense. However, as of today, ILCs are thought to represent the master key to innate immune responses on mucosal surfaces, and are the focus of this review as discussed below.
Innate lymphoid cells
Introduction
The term innate lymphoid cell refers to well-established, recently identified populations that seem to share a common origin and derive from Id2-dependent lymphoid progenitors (19-23). ILCs are defined by three major characteristics -absence of antigen-specific receptors and memory function, lack of myeloid phenotypic markers, and lymphoid morphology (24). In fact, these cells do not directly recognize pathogens but respond to cytokine pattern changes induced by pathogenic infection (21,22,25,26). A prototypal population of innate lymphoid cells is that of natural killer (NK) cells and lymphoid tissue inducer (LTi) cells. NK cells mediate in early immune responses to viruses, and are involved in the protection against cancer cells (27,28). LTi cells are key for lymph node formation during embryogenesis (27,29).
Recently, other ILC populations have been identified that, as is the case with NK and LTi cells, are also dependent on γc and ID2 for their development. These various ILC populations have different cytokine production patterns that reflect the different subpopulations of Th lymphocytes (22). For example, following activation, some ILC populations secrete Th17-related cytokines, including interleukine (IL)-17 and IL-22, whereas other ILC subsets secrete Th2 cytokines (IL-5 and IL-13) (22).
ILC populations may play effector roles in early immune responses against pathogens (30,31), in addition to their contribution to mucous tissue repair (32,33), anatomical contention of the commensal flora, and maintenance of epithelial integrity (34). On the other hand, ILC subpopulations involved in several inflammatory conditions have been found. For example, IL-17- and interferon (IFN)-γ-producing ILCs have been shown to be mediators of colitis in a murine IBD (inflammatory bowel disease) model (35), whereas Th2 cytokine-producing ILCs give rise to pulmonary inflammation in some allergic asthma models (36).
Classification of innate lymphoid cells
The recent identification of various ILC subsets has led to a new classification for them. Currently, the most widely accepted classification is that proposed by Spits et al. (24), which is based on cell phenotype and functional characteristics, and includes three groups (Fig. 2):
- Group 1 ILCs, which include classic NK cells (28,37-39). These cells rely on T-bet and IL-15 expression for optimal differentiation and function (40,41). Their stimulation may also results from IL-12 and IL-18 signaling, and NCR (natural cytotoxicity receptor) expression. Activation results in proinflammatory cytokine production, including IFNg and TNFa, or perforin and granzyme release to induce lysis in target cells, key processes for tumor suppression and immunity against certain intracellular pathogens (37-39,42). Therefore, NK cells share both homeostatic and functional similarities with CD8+ T-cells.
Another group 1 subpopulation has been recently identified in humans that differs from NK cells and produces IFNg but no Th17 or Th2 cytokines (43). These are known as ILC1s to differentiate them from classic NK cells. In humans they are characterized by their secretion of IFNg, lack of KIT (CD117) expression, and high levels of T-bet and low levels of RORgt expression (43). They are thought to be RORgt+ ILCs (included in group 3) that increase T-bet and IFNγ production, and lose RORγt when under stimulation (35,43,44).
- Group 2 ILCs require IL-7 for their development (31) and produce Th2 cytokines in response to stimulation with IL-25 (IL-17E) (45,46), IL-33 (31), and TSLP (thymic stromal lympho-poietin) (47). Only one subset has been identified in humans. These are cells characterized by their expression of ST2 (also known as IL-1RL1), which is a component of the IL-33 receptor, and of IL-17RB, which is a subunit of the IL-25 receptor (33,48). Furthermore, human ILC2s express CRTH2 (prostaglandin D2 and chemoattractant receptor-homologous molecule 2 expressed by Th2 lymphocytes) and CD161. Only human ILC2s can produce IL-4, in contrast to mouse ILC2s (48). The development of these cells is mediated by the GATA3 transcription factor (49).
- Group 3 ILCs are defined by their ability to produce IL-17A and/or IL-22, whether with NCR expression or otherwise. As with Th17 cells, the development and function of these cells are dependent upon transcription factor RORγt and IL-7 receptor. Prototypical cells in this group include LTi cells, which are crucial for the formation of secondary lymphoid organs during embryogenesis. An effector role has also been suggested for these cells within innate immunity because of their IL-17A and IL-22 secretion following stimulation (50). Surface markers on human LTi cells are identical to those in their mouse counterparts except for CD4, which is expressed by most murine but no human LTi cells (51). On the other hand, human LTi cells express NKp46 as do their murine counterparts, but also other NCRs such as NKp30 and NKp44 (30). This group 3 ILC population is heterogeneous and includes various types of cells that produce IL-17A and/or IL-22, or IL-17A, IL-22 and IFNg; these could be considered distinct stable cell subsets or, alternatively, different forms of only one cell type with plastic capabilities (24).
Functions of innate lymphoid cells
Several ILC subpopulations have been identified in human tissues, both healthy and with various conditions, including secondary lymphoid organs, peripheral blood, lungs, and gut (26,52,53). NK cells have been most thoroughly studied, and changes in their numbers and/or functions have been associated with some diseases (54). Furthermore, NK cells play a major role in various conditions such as viral infection, inflammatory disorders, pregnancy, cancer, and bone marrow transplantation (37,54). Similarly, group 2 ILCs have been recently identified that secrete IL-5 and IL-13 in the blood of healthy individuals, in the lungs and bowel of both fetal and adult donors, in the bronchoalveolar fluid of lung transplant recipients, and in the nasal polyps of patients with chronic rhinosinusitis (33,48).Group 3 ILCs, which secrete IL-17A and IL-22, are seen in the secondary lymphoid tissues and the intestinal tissue of both fetal and adult donors (30,34,51). In co-culture experiments with mesenchymal stem cells from murine models RORγt+ ILCs have been seen to promote the expression of adhesion molecules involved in lymphoid organogenesis (51), and the proliferation of intestinal epithelial cells (30).
ILCs play their role mainly by secreting cytokines, but they may also modify the adaptive response mediated by B cells and T cells via cell-cell interactions. An example of the above is the ability of LTi cells to interact with CD4+ memory cells through OX40L and CD30L (55,56). Similarly, ILCs might directly interact with the intestinal bacterial flora. While the detection of certain specific bacterial components is vital for the development of adaptive immune system cells, this requirement seems less important regarding the development of innate cells, including macrophages and NK cells, given their presence in bacteria-free mice (5). However, recent studies have shown that the bacterial flora directly regulates the role of innate cells, just as the innate immune system directly acts on the composition of the bacterial flora (42,57).
Several studies have also characterized the contribution of RORγt+ ILCs to the pathogenesis and progression of some diseases where host-commensal flora interactions are impaired. Geremia et al. (58) observed a substantial increase in NCR-RORγt+, IL-17A- and IL-22-producing ILCs in the inflamed intestinal tissues of patients with Crohn's disease (CD). The potential significance of changes in the balanced production of IL-22 between NCR+ ILCs and NCR-RORγt ILCs in patients with IBD may be explained by a differential IL-17A expression in these two subsets (26,53,59), given the proinflammatory role of IL-17A versus the protective function of IL-22 in the bowel mucosa (60).
NKT cells
Natural killer T (NKT) cells are a subset of T cells that express receptors characteristic of T and NK cells (61-65). As NK cells, they are included among group 1 ILCs (24) and contain perforins and granzymes allowing them to take part in the innate immune response (61-63). In contrast to conventional T cells, which recognize peptides bound by major histocompatibility complex (MHC) class I or class II molecules, NKT cells recognize lipidic and glycolipidic structures bound by CD1d molecules (66). They may be found mainly in the liver, spleen and bone marrow, and their development is dependent upon the thymus. In the bowel, various NKT subsets have been described among IELs and in the LP (66). Intestinal NKT-cell activationcontributes to mucosal immunity against both pathogenic and commensal bacteria. Furthermore, uncontrolled or inadequate NKT-cell activation may contribute to the pathogenesis of intestinal inflammatory diseases (67).
NKT cells were initially defined by their co-expression of TCRab (T-cell receptor) and NK-cell receptors, particularly NK1.1 in selected mouse strains and CD161 in humans (64). However, conventional T cells may express NK-like receptors (61,68). Today, the most widely accepted classification is the one suggested by Wingender et al. (69), which is based on TCR composition and recognized antigen-presenting molecule, giving four distinct populations (Table I). The first two sets have a canonic or invariant TCR and interact with non-polymorphic molecules similar to MHC class I (69). The first population, designated invariant NKT or iNKT cells, includes cells that express a semi-invariant TCR composed of Vα14 - Jα18 and Vβ8.2, -7, or -2 chains in mice, or the homologous Vα24 - Jα18 and Vβ11 chains in humans (63). The second subset includes mucosal NKT or mNKT cells, characterized by their expression of an invariant TCR Va7.2 in mice, and the homologous Va19 in humans (66). A third group, called variant NKT or vNKT cells, includes NKT cells reactive to CD1d but with no fixed-composition TCR (70,71). The fourth group, that of xNKT or NKT-like cells, is most heterogeneous and includes all T cells expressing NK-like receptors. This group includes T cells not dependent on CD1d expression for their development or reactivity, and that may recognize lipids presented by CD1 molecules (CD1a, -b, -c); antigen recognition, however, is not restricted to lipids but also applies to peptides in the setting of class-I or class-II MHC molecules (69).
As discussed in this review, ILCs are of utmost importance for intestinal immunity. To gain a deeper insight into this topic, we shall now focus primarily on NKT cells within ILC1 group, particularly on the invariant NKT subset, and their relevance for the immune response in the gastrointestinal tract.
Invariant NKT or iNKT cells
Biology of iNKT cells
So-called invariant NKT or iNKT cells represent the most numerous and most widely studied fraction of NKT cells. As discussed above, these cells are characterized by their having a TCR made up with Vα14 - Jα18 and Vβ8.2, -7, or -2 chains in mice, or their homologous Vα24 - Jα18 and Vβ11 chain in humans (63). iNKT cells recognize glycolipidic structures presented by CD1d, a molecule homologous to class-I MHC (63). While there is a high degree of cross-reactivity between species, since mouse iNKT cells may recognize antigens presented human CD1d molecules, and vice versa, significant differences are also present between iNKT cells in both species (72).
As occurs with conventional T-cells, NKT cells develop from thymic precursors. Immature CD4+CD8+ T cells derived from these precursors give rise to the NKT cell lineage that depends on signals via CD1d molecules expressed by cortical thymocytes, which may present self-glycolipids (73). As iNKT cells differentiate in the thymus, they express a surface marker pattern (CD69+, CD44high, CD11ahigh, CD62low, CD122+) that is typically associated with memory or activated T cells (69). Following activation, iNKT cells rapidly develop cytotoxic activity, and produce both Th1 (IFNγ and TNFα) and Th2 (IL-4, IL-10, and IL-13) cytokines (74); also, some iNKTs have been recently shown to secrete IL-17 (74,75). iNKT cells may be involved in the early stages of a great variety of immune responses, ranging from oral tolerance to autoimmunity development, including responses to both pathogenic and tumor agents (64,68).
Following their development in the thymus, a major fraction of iNKT cells stays there and the rest migrate to peripheral sites, where they make up a relevant T-cell subset in the bone marrow, spleen, blood and liver, being less common in lymph nodes (67). Some of these cells reach the intestinal and pulmonary mucosa. Interestingly, iNKT cells are less common in most human versus murine organs, and their prevalence varies greatly between subjects because of yet unknown reasons (67). Recent studies show that ageing results in a rapid and significant decrease in peripheral blood iNKT cells associated with an increased proportion of CD4+ iNKTs and fewer double-negative iNKT cells. Furthermore, iNKT cells from older subjects secrete more IL-4 when compared to younger individuals (76). These results may also explain age-related Th1/Th2 cytokine profile changes and shed light on the mechanisms of immunosenescence. Given the significance of iNKT cells for immune response initiation and regulation, these results may also help in understanding the increased incidence of both infections and malignancies, as well as the increased severity of autoimmune conditions, in the elderly (76,77).
Several lipidic or glycolipidic antigens have been identified, which may be presented by CD1d and activate iNKT cells. KRN7000, an α-galactosylceramide (α-GalCer) that was originally discovered in a sea sponge and possesses anti-metastatic properties in mice, is prototypical (78). α-GalCer likely derives from the Sphingomonas bacteria that colonize this sponge (79-81). The current hypothesis establishes that iNKT cells can recognize natural glycolipidic structures in several bacterial pathogens, including Borrelia burgdorferi, Ehrlicha bacteria, Streptococcus pneumoniae (79) and Bacteroide fragilis (82).
The effector phenotype of iNKT cells and their constitutive expression of IL-4 and IFNγ mRNAs suggests that these cells undergo strong antigenic stimulation during differentiation (64). In this regard, it is suggested that mature iNKT cells may, under certain circumstances, recognize CD1d-bound endogenous glycolipids. As of today, the best such candidate is a glycosphingolipid that is present in the lysosomes of some cells -isoglobotrihexosylceramide (iGb3) (83). However, recent data raise doubts regarding the role of iGb3 as single endogenous antigen in iNKT-cell development, as mice deficient in iGb3- synthase have been found with a normal count of functional iNKT cells (84). A third class of iNKT ligands has been identified from dietary lipids used as emulsifiers and thickeners. These compounds are similar to glycolipids found in the walls of some bacteria, including the genus Mycobacteria (85), and may activate iNKT cells. The affinity of iNKT TCR for CD1d-bound antigens is not always enough to predict cytokine response types (Th1 or Th2). Available data suggest that response polarization in iNKT cells is determined by antigen-CD1d binding strength, cell surface complex longevity, and APC type (86).
iNKT cells may also be directly activated by proinflammatory cytokines such as IFNγ, IL-12, and IL-18, either alone or combined, which are produced by macrophagesand DCs early on after bacterial or viral infection (87). While, in some instances, iNKT cells have been shown to require the recognition of CD1d-presented self-endogenous ligands for activation in this setting, direct activation most often ensues without TCR-related antigen recognition. This type of direct activation prompts iNKT cells to produce IFNγ but not IL-4 (87).
iNKT cells in the human bowel
The percentage of iNKT cells present in the human bowel is controversial. To date, these cells may only be unequivocally detected through mRNA quantification for invariant TCR chains or by flow cytometry CD1d-αGalCer tetramers (69). Via flow cytometry analysis, the proportions of CD161-expressing T cells to total lymphocytes are: 50-70 % of IELs in the small bowel (88,89), 40-45 % of IELs in the large bowel (88,89), and 9 % of lymphocytes in the large bowel LP (90). However, only 1.6 -1.7 % of intestinal CD3ε/CD161 cells express Va24 (88,89), and immunohistochemical data suggest that most of these cells reside in the LP (91). By using CD1d-αGalCer tetramers for flow cytometry the proportion of iNKT cells in the human bowel is estimated to be 0.4 % of all T cells, with the LP being their primary site (90). However, despite their low numbers these cells can produce huge amounts of cytokines following activation with α-GalCer (89).
CD1d expression is prerequisite for antigen-specific iNKT-cell activation (86). This molecule resembles those in the class-I MHC, consisting of a light chain (β2-microglobulin) covalently bonded with a heavy chain (92), and is structurally related to HLA-A, HLA-B and HLA-C molecules in IECs (62). CD1d is expressed by professional APCs, including dendritic cells, macrophages, and B cells, and non-professional APCs, including liver cells and IECs (93-95).
A controversial aspect of human IECs is CD1d expression, as most of these cells express a CD1d form not associated with β2-microglobulin, mainly intracellular, and with surface expression restricted to the apical pole (93,96-98). While its function is not clear, and no evidence exists on the recognition of this CD1d form by iNKTs, it has been suggested that some T cells, probably vNKT cells, do recognize it (93,99,100). IECs weakly express native CD1d, preferably on their basal pole (98). However, these human IECs may activate iNKT cells in vitro via CD1d (101,102). A CD1d-dependent feedback pathway has also been found to play a role in IL-10 signaling and production in the intestinal mucosa via IEC activation (86). The abundance of CD1d in the intestine and the power of CD1d-dependent iNKT activation suggest their involvement in intestinal homeostasis, bacterial regulation in the bowel, and protection against pathogens such as Salmonella typhimurium and Toxoplasma gondii (103-105).
The immune system's ability to discriminate between pathogenic and non-pathogenic antigens is the basis of immune tolerance. A relevant component of oral tolerance against dietary and saprophytic flora antigens is represented by regulatory intestinal cells, including IL-10-secreting regulatory T (Treg) cells, tolerogenic DCs, and iNKT cells (106). Treg lymphocytes may inhibit iNKT activity by cell-to-cell contact (107), whereas iNKT cells increase regulatory intestinal cell activity by producing cytokines such as IL-2 and TGFβ (108). Treg lymphocytes can suppress Th1 and Th2 responses (109), whereas iNKTs suppress CD8+ T-cell activation, henceTh1 responses, and either increase or suppress Th2 responses (110,111). Furthermore, iNKT cells can induce immature DC maturation to tolerogenic DCs (106). When tolerance mechanisms fail, an inappropriate immune response against dietary and saprophytic flora antigens ensues, giving rise to inflammatory bowel conditions such as coeliac disease (CoD) or IBD, respectively.
The role of iNKT cells in intestinal diseases
Few studies exist on iNKT-cell activation in patients with IBD; however, studies in murine models and our current understanding of iNKT-cell biology suggest a relevant role. Furthermore, an increase in CD1d expression has been seen in the terminal ileal epithelium of patients with CD, and in the involved cecal area of patients with ulcerative colitis (UC), which might increase the recruitment of CD1d-dependent proinflammatory cells and bring about the destruction of the intestinal mucosa in IBD (112). However, a more recent study suggests that, in contrast to IECs in healthy individuals, IECs in patients with IBD do not express CD1d, which results in abnormal NKT-cell regulation (101).
Clinical studies have shown a significant reduction in peripheral blood iNKT cells in patients with CD, assessed as Vα24/Vβ11+ cells or cells reactive against tetramer CD1d-αGalCer (113,114), as well as a reduced expression of Vα24 and a decrease in iNKT-cell numbers in the bowel (113). However, aberrant iNKT-cell activation is likely given that these cells may secrete huge amounts of IFNg through IL-12 mediation (89). Interestingly, IECs in the terminal ileum, the primary site for CD, show numerous lipid-containing lysosomes that may act as potent iNKT-cell activators (115,116). IL-13-producing NKT cells have been identified in UC patients. In contrast with the murine oxazolone-induced colitis model, these cells do not express the invariant TCR Vα24 chain, hence they are no iNKTs despite their CD1d-dependent activation (117).
A potential involvement of iNKT cells as regulatory or protective agents in IBD has also been posited. In the murine DSS (dextran sodium sulfate)-induced colitis model the use of the iNKT-cell activator αGalCer (118,119), or its analogue OCH (120), has been seen to result in great improvement. This is due to iNKT-cell polarization towards a Th2 profile with an increase in IL-4 and IL-10 production, and a decrease in IFNγ (120).
The innate and adaptive characteristics of iNKT cells, and their ability to produce huge amounts of IL-4 and IFNγ, also suggest their involvement in CoD (91,114,121). The various studies performed to quantify circulating or intestinal iNKT cells in celiac patients have yielded controversial findings (91,114). In some cases a decreased number of iNKT cells have been found in the duodenum of these patients (91,122). However, prior findings by our team show an increase of these cells only in the epithelial compartment during active disease. These findings, together with the correlation between Vα24 mRNA expression and total intraepithelial iNKT-cell count, suggest that iNKT cells may be a source of IFNγ in CoD (manuscript awaiting publication). Today, natural ligands of iNKT cells are thought to include glycolipids in the cytoplasm of enterocytes, which are released into the extracellular matrix after apoptosis or necrosis (123) in the presence of IFNγ and IL-15, as occurs in CoD (124), while IL-15 plays a central role in the activation and biologic functions of these cells (125). Finally, a decrease in total circulating iNKT-cell numbers has been seen in refractory CoD; however, whether this is a cause or a result of disease malignant progression remains unknown (126).
A predominance of the CD4+ regulatory phenotype versus the proinflammatory phenotype of intestinal iNKT cells has been recently described in healthy individuals. However, HIV (human immunodeficiency virus) infection results in a decrease in this intestinal iNKT population that directly correlates with poorer immune response, the hallmark of disease progression (127).
Multiple studies have shown the high potential of iNKT cells to initiate an effective anti-tumor response. The activation of these cells as a result of αGalCer stimulation has demonstrated anti-tumor effects in several experimental models and in spontaneous skin, liver, lung and intestine metastatic models, including colon-26 adenocarcinoma, EL-4 T-cell lymphoma, sarcoma, melanoma, and carcinoma (128-130). αGalCer rapidly activates iNKT cells and apoptosis ensues, which suggests that iNKT cells initiate the primary antitumor response by direct cytotoxicity, and then activate more persistent immune mechanisms aimed at the destruction of tumor cells (121).
Conclusion
The innate immune response is key for the maintenance of epithelial integrity, homeostasis, and early response to pathogens in the intestinal mucosa. Innate lymphoid cells seem to be key components of such response. Group 1 ILCs include NKT cells, a subset with various peculiarities that play an active role in this response. The most important fraction in this subpopulation is iNKT cells. Due to their effector phenotype and great capability to produce huge amounts of cytokines upon activation, their taking part in several immune processes has been suggested, including intestinal homeostasis, defense against tumors and several pathogens, and an active role in the development of a number of inflammatory conditions. Their activation results from the recognition of glycolipids presented by CD1d. Although the source of these immunogenic glycolipids that trigger active, iNKT-mediated responses remains unknown in humans, a number of both endogenous and exogenous origins may be posited. Among these, intracellular lipids from apoptotic enterocytes released during inflammation, dietary glycolipids modified by non-physiological enzymes, and glycolipids from bacteria within the intestinal lumen stand out. Identifying the specific role of these cells in each process, as well as their specific ligands, is key for the development of promising therapies for the treatment of intestinal inflammatory diseases.
References
1. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336(6086):1268-73. [ Links ]
2. Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, Rognum TO, et al. Immunobiology and immunopathology of human gut mucosa: Humoral immunity and intraepithelial lymphocytes. Gastroenterology 1989;97(6):1562-84. [ Links ]
3. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489(7415):231-41. [ Links ]
4. Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol 2011;32(6):256-64. [ Links ]
5. Goto Y, Ivanov, II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunology and Cell Biology 2013;91(3):204-14. [ Links ]
6. Granucci F, Zanoni I, Ricciardi-Castagnoli P. Central role of dendritic cells in the regulation and deregulation of immune responses. Cellular and molecular life sciences: CMLS 2008;65(11):1683-97. [ Links ]
7. Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: Role of dendritic cells and toll-like receptors. Critical Reviews in Immunology 2006;26(4):291-306. [ Links ]
8. Perera PY, Lichy JH, Waldmann TA, Perera LP. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect 2012;14(3):247-61. [ Links ]
9. Bernardo D. Human intestinal dendritic cells as controllers of mucosal immunity. Rev Esp Enferm Dig 2013;105(5):279-90. [ Links ]
10. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 2007;8(10):1086-94. [ Links ]
11. Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunology and Cell Biology 2013;91(3):232-9. [ Links ]
12. Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2(4):361-7. [ Links ]
13. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity 2009;31(3):513-25. [ Links ]
14. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009;206(13):3101-14. [ Links ]
15. Mann ER, Landy JD, Bernardo D, Peake ST, Hart AL, Al-Hassi HO, et al. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett 2013;150(1-2):30-40. [ Links ]
16. Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. Journal of Innate Immunity 2011;3(6):550-64. [ Links ]
17. Svensson-Frej M. Immunobiology of intestinal eosinophils - a dogma in the changing? Journal of Innate Immunity 2011;3(6):565-76. [ Links ]
18. Johnsson M, Bove M, Bergquist H, Olsson M, Fornwall S, Hassel K, et al. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. Journal of Innate Immunity 2011;3(6):594-604. [ Links ]
19. Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol 2011;187(11):5505-9. [ Links ]
20. Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol 2012;13(3):229-36. [ Links ]
21. Veldhoen M, Withers DR. Immunology. Innate lymphoid cell relations. Science 2010;330(6004):594-5. [ Links ]
22. Spits H, Di Santo JP. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat Immunol 2011;12(1):21-7. [ Links ]
23. Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 2012;37(4):634-48. [ Links ]
24. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells -- a proposal for uniform nomenclature. Nat Rev Immunol 2013;13(2):145-9. [ Links ]
25. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011;474(7351):298-306. [ Links ]
26. Spits H, Cupedo T. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012;30:647-75. [ Links ]
27. Pearson C, Uhlig HH, Powrie F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol 2012;33(6):289-96. [ Links ]
28. Kiessling R, Klein E, Pross H, Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol 1975;5(2):117-21. [ Links ]
29. Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997;7(4):493-504. [ Links ]
30. Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009;457(7230):722-5. [ Links ]
31. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010;463(7280):540-4. [ Links ]
32. Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol 2008;9(6):667-75. [ Links ]
33. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011;12(11):1045-54. [ Links ]
34. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 2012;336(6086):1321-5. [ Links ]
35. Buonocore S, Ahern PP, Uhlig HH, Ivanov, II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010;464(7293):1371-5. [ Links ]
36. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol 2011;12(7):631-8. [ Links ]
37. Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu Rev Immunol 1999;17:189-220. [ Links ]
38. Di Santo JP. Natural killer cells: Diversity in search of a niche. Nat Immunol 2008;9(5):473-5. [ Links ]
39. Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004;22:405-29. [ Links ]
40. Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012;36(1):55-67. [ Links ]
41. Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity 2004;20(4):477-94. [ Links ]
42. Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012;37(1):171-86. [ Links ]
43. Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013;14(3):221-9. [ Links ]
44. Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012;37(4):674-84. [ Links ]
45. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001;15(6):985-95. [ Links ]
46. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. J Immunol 2002;169(1):443-53. [ Links ]
47. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012;36(3):451-63. [ Links ]
48. Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 2011;12(11):1055-62. [ Links ]
49. Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 2012;37(4):649-59. [ Links ]
50. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, II, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 2009;206(1):35-41. [ Links ]
51. Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 2009;10(1):66-74. [ Links ]
52. Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol. 2012;24(3):284-9. [ Links ]
53. Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 2011;12(5):383-90. [ Links ]
54. Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol 2006;118(1):1-10. [ Links ]
55. Kim MY, Toellner KM, White A, McConnell FM, Gaspal FM, Parnell SM, et al. Neonatal and adult CD4+ CD3- cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15). J Immunol 2006;177(5):3074-81. [ Links ]
56. Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, et al. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity 2003;18(5):643-54. [ Links ]
57. Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012;37(1):158-70. [ Links ]
58. Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 2011;208(6):1127-33. [ Links ]
59. Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 2009;31(1):15-23. [ Links ]
60. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med 2010;207(6):1293-305. [ Links ]
61. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007;25:297-336. [ Links ]
62. Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol 2004;22:817-90. [ Links ]
63. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What's in a name? Nat Rev Immunol 2004;4(3):231-7. [ Links ]
64. Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 2005;23:877-900. [ Links ]
65. Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol 2007;19(3):354-64. [ Links ]
66. Middendorp S, Nieuwenhuis EE. NKT cells in mucosal immunity. Mucosal Immunol 2009;2(5):393-402. [ Links ]
67. Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell and Tissue Research 2011;343(1):43-55. [ Links ]
68. Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nature reviews Microbiology 2007;5(6):405-17. [ Links ]
69. Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Gastrointest Liver Physiol 2008;294(1):G1-8. [ Links ]
70. Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. Journal of Cellular Physiology 2009;218(2):246-50. [ Links ]
71. Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med 1999;189(1):103-10. [ Links ]
72. Treiner E, Lantz O. CD1d- and MR1-restricted invariant T cells: Of mice and men. Curr Opin Immunol 2006;18(5):519-26. [ Links ]
73. Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol 2005;5(6):459-71. [ Links ]
74. Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A 2008;105(32):11287-92. [ Links ]
75. Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med 2007;204(5):995-1001. [ Links ]
76. Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Experimental Gerontology 2007;42(8):719-32. [ Links ]
77. Peralbo E, DelaRosa O, Gayoso I, Pita ML, Tarazona R, Solana R. Decreased frequency and proliferative response of invariant Valpha24Vbeta11 natural killer T (iNKT) cells in healthy elderly. Biogerontology 2006;7(5-6):483-92. [ Links ]
78. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997;278(5343):1626-9. [ Links ]
79. Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005;434(7032):520-5. [ Links ]
80. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 2005;434(7032):525-9. [ Links ]
81. Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol 2005;35(6):1692-701. [ Links ]
82. Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, et al. Production of alpha-galactosylceramide by a prominent member of the human gut microbiota. PLoS biology 2013;11(7):e1001610. [ Links ]
83. Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 2004;306(5702):1786-9. [ Links ]
84. Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A 2007;104(14):5977-82. [ Links ]
85. Traunmuller F. Etiology of Crohn's disease: Do certain food additives cause intestinal inflammation by molecular mimicry of mycobacterial lipids? Medical Hypotheses 2005;65(5):859-64. [ Links ]
86. van Dieren JM, van der Woude CJ, Kuipers EJ, Escher JC, Samsom JN, Blumberg RS, et al. Roles of CD1d-restricted NKT cells in the intestine. Inflamm Bowel Dis 2007;13(9):1146-52. [ Links ]
87. Lawson V. Turned on by danger: activation of CD1d-restricted invariant natural killer T cells. Immunology 2012;137(1):20-7. [ Links ]
88. Iiai T, Watanabe H, Suda T, Okamoto H, Abo T, Hatakeyama K. CD161+ T (NT) cells exist predominantly in human intestinal epithelium as well as in liver. Clin Exp Immunol 2002;129(1):92-8. [ Links ]
89. O'Keeffe J, Doherty DG, Kenna T, Sheahan K, O'Donoghue DP, Hyland JM, et al. Diverse populations of T cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer. Eur J Immunol 2004;34(8):2110-9. [ Links ]
90. Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 2004;113(10):1490-7. [ Links ]
91. Grose RH, Cummins AG, Thompson FM. Deficiency of invariant natural killer T cells in coeliac disease. Gut 2007;56(6):790-5. [ Links ]
92. Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science 1997;277(5324):339-45. [ Links ]
93. Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, et al. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science 1994;265 (5169):259-62. [ Links ]
94. Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science 1990;250(4981):679-82. [ Links ]
95. Blumberg RS, Terhorst C, Bleicher P, McDermott FV, Allan CH, Landau SB, et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol 1991;147(8):2518-24. [ Links ]
96. Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of beta2-microglobulin. J Biol Chem 1999;274(14):9289-95. [ Links ]
97. Kim HS, Colgan SP, Pitman R, Hershberg RM, Blumberg RS. Human CD1d associates with prolyl-4-hydroxylase during its biosynthesis. Mol Immunol 2000;37(14):861-8. [ Links ]
98. Somnay-Wadgaonkar K, Nusrat A, Kim HS, Canchis WP, Balk SP, Colgan SP, et al. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a beta2-microglobulin-associated form. Int Immunol 1999;11(3):383-92. [ Links ]
99. Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med 1995;182(4):993-1004. [ Links ]
100. Panja A, Blumberg RS, Balk SP, Mayer L. CD1d is involved in T cell-intestinal epithelial cell interactions. J Exp Med 1993;178(3):1115-9. [ Links ]
101. Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis 2007;13(3):298-307. [ Links ]
102. Pellicci DG, Hammond KJ, Coquet J, Kyparissoudis K, Brooks AG, Kedzierska K, et al. DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells. J Immunol 2005;175(7):4416-25. [ Links ]
103. Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest 2009;119(5):1241-50. [ Links ]
104. Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol 2005;35(7):2100-9. [ Links ]
105. Ronet C, Darche S, Leite de Moraes M, Miyake S, Yamamura T, Louis JA, et al. NKT cells are critical for the initiation of an inflammatory bowel response against Toxoplasma gondii. J Immunol 2005;175(2):899-908. [ Links ]
106. La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol 2006;27(7):322-7. [ Links ]
107. Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res 2003;63(15):4516-20. [ Links ]
108. Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+CD25+ regulatory T cells? Eur J Immunol 2005;35(4):1193-200. [ Links ]
109. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531-62. [ Links ]
110. Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 2001;413(6855):531-4. [ Links ]
111. Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity 2002;17(6):725-36. [ Links ]
112. Page MJ, Poritz LS, Tilberg AF, Zhang WJ, Chorney MJ, Koltun WA. Cd1d-restricted cellular lysis by peripheral blood lymphocytes: relevance to the inflammatory bowel diseases. The Journal of Surgical Research 2000;92(2):214-21. [ Links ]
113. Grose RH, Thompson FM, Baxter AG, Pellicci DG, Cummins AG. Deficiency of invariant NK T cells in Crohn's disease and ulcerative colitis. Dig Dis Sci. 2007;52(6):1415-22. [ Links ]
114. van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol 2001;100(2):144-8. [ Links ]
115. Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH, Jr. Freeze-fracture analysis of epithelial cell lysosomal inclusions in Crohn's disease. Ultrastructural pathology 1984;6(1):39-44. [ Links ]
116. Thyberg J, Graf W, Klingenstrom P. Intestinal fine structure in Crohn's disease. Lysosomal inclusions in epithelial cells and macrophages. Virchows Archiv A, Pathological Anatomy and Histology 1981;391(2):141-52. [ Links ]
117. Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 2002;17(5):629-38. [ Links ]
118. Numata Y, Tazuma S, Ueno Y, Nishioka T, Hyogo H, Chayama K. Therapeutic effect of repeated natural killer T cell stimulation in mouse cholangitis complicated by colitis. Dig Dis Sci 2005;50(10):1844-51. [ Links ]
119. Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology 2000;119(1):119-28. [ Links ]
120. Ueno Y, Tanaka S, Sumii M, Miyake S, Tazuma S, Taniguchi M, et al. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of valpha14 natural killer T cells in mice. Inflamm Bowel Dis 2005;11(1):35-41. [ Links ]
121. van der Vliet HJ, Molling JW, von Blomberg BM, Nishi N, Kolgen W, van den Eertwegh AJ, et al. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin Immunol 2004;112(1):8-23. [ Links ]
122. Calleja S, Vivas S, Santiuste M, Arias L, Hernando M, Nistal E, et al. Dynamics of non-conventional intraepithelial lymphocytes-NK, NKT, and gammadelta T-in celiac disease: relationship with age, diet, and histopathology. Dig Dis Sci 2011;56(7):2042-9. [ Links ]
123. Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol 2011;12(12):1202-11. [ Links ]
124. Sarra M, Cupi ML, Monteleone I, Franze E, Ronchetti G, Di Sabatino A, et al. IL-15 positively regulates IL-21 production in celiac disease mucosa. Mucosal Immunol 2013;6(2):244-55. [ Links ]
125. Gill N, Rosenthal KL, Ashkar AA. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J Virol 2005;79(7):4470-8. [ Links ]
126. Bernardo D, van Hoogstraten IM, Verbeek WH, Pena AS, Mearin ML, Arranz E, et al. Decreased circulating iNKT cell numbers in refractory coeliac disease. Clin Immunol 2008;126(2):172-9. [ Links ]
127. Ibarrondo FJ, Wilson SB, Hultin LE, Shih R, Hausner MA, Hultin PM, et al. Preferential depletion of gut CD4-expressing iNKT cells contributes to systemic immune activation in HIV-1 infection. Mucosal Immunol 2013;6(3):591-600. [ Links ]
128. Nakui M, Ohta A, Sekimoto M, Sato M, Iwakabe K, Yahata T, et al. Potentiation of antitumor effect of NKT cell ligand, alpha-galactosylceramide by combination with IL-12 on lung metastasis of malignant melanoma cells. Clinical & experimental Metastasis 2000;18(2):147-53. [ Links ]
129. Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, et al. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res 1998;58(6):1202-7. [ Links ]
130. Nakagawa R, Serizawa I, Motoki K, Sato M, Ueno H, Iijima R, et al. Antitumor activity of alpha-galactosylceramide, KRN7000, in mice with the melanoma B16 hepatic metastasis and immunohistological study of tumor infiltrating cells. Oncology Research 2000;12(2):51-8. [ Links ]
![]() Correspondence:
Correspondence:
David Bernardo.
Antigen Presentation Research Group.
Imperial College London.
Northwick Park &
St Mark's Campus, Level 7W,
St. Mark's Hospital, Watford Road,
Harrow, HA1 3UJ, United Kingdom
e-mail: d.bernardo-ordiz@imperial.ac.uk
Received: 17-02-2014
Accepted:10-03-2014











 texto en
texto en