Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 no.8 Madrid dic. 2014
Effect of necrosectomy and vacuum-assisted closure (VAC) on mitochondrial function and oxidative stress markers in severe acute pancreatitis
Efecto de la necrosectomía y el sistema de cierre asistido al vacío (VAC) sobre función mitocondrial y marcadores de estrés oxidativo en pancreatitis aguda severa
Alejandra Guillermina Miranda-Díaz1, José Manuel Hermosillo-Sandoval2, Carlos Alberto Gutiérrez-Martínez3, Adolfo Daniel Rodríguez-Carrizalez1, Luis Miguel Román-Pintos1, Ernesto Germán Cardona-Muñoz1, Fermín Paul Pacheco-Moisés4 and Óscar Arias-Carvajal1
1Department of Physiology. Centro Universitario de Ciencias de la Salud. Universidad de Guadalajara. Guadalajara, Jalisco. Mexico.
2Department of General Surgery and
3Intensive Care Unit. Hospital de Especialidades. Centro Médico Nacional de Occidente. Instituto Mexicano del Seguro Social. Guadalajara, Jalisco. Mexico.
4Department of Chemistry. Universidad de Guadalajara. Guadalajara, Jalisco. Mexico
ABSTRACT
Background: Severe acute pancreatitis (SAP) is associated with high morbidity and mortality.
Objective: To evaluate whether necrosectomy, alone or combined with vacuum-assisted closure (VAC), has any additional beneficial effects on mitochondrial function and/or oxidative stress markers in SAP.
Methods: Patients with SAP, APACHE II score > 8, and inadequate response to management in an intensive care unit were included in a prospective observational study. Sixteen underwent necrosectomy and 24 underwent necrosectomy plus VAC every 48 h. Patients were then categorized as survivors or deceased. Submitochondrial membrane fluidity of platelets and F0F1-ATPase hydrolysis were measured to represent mitochondrial function. Oxidative/nitrosative stress was measured using lipoperoxides (LPOs), nitric oxide (NO), erythrocyte membrane fluidity, and total antioxidant capacity (TAC).
Results: Membrane fluidity in submitochondrial particles of platelets remained significantly increased throughout the study, and then eventually rised in deceased patients managed with necrosectomy + VAC vs. survivors (p < 0.041). Hydrolysis was significantly increased from baseline to endpoint in all patients, predominating in those who died after management with necrosectomy (p < 0.03). LPO increased in all patients, and necrosectomy was more efficient for the eventual decrease in survivors (p < 0.039). NO was found to be increased for the baseline-endpoint result among both survivors and deceased patients with both management options. Erythrocyte membrane fluidity was increased in survivors managed with necrosectomy + VAC, and eventually returned to normal (p < 0.045). TAC was found to be consumed in all patients for the duration of the study.
Conclusions: Mitochondrial dysfunction and oxidative/nitrosative stress with significant systemic antioxidant consumption were found. Necrosectomy was more efficient and better cleared LPOs. Necrosectomy + VAC improved erythrocyte membrane fluidity and increased survival.
Key words: Acute pancreatitis. Mitochondrial dysfunction. Oxidative stress. Severe acute pancreatitis.
RESUMEN
Antecedentes: la pancreatitis aguda severa (PAS) se asocia con alta morbilidad y mortalidad.
Objetivo: evaluar si la necrosectomía sola o necrosectomía + el sistema de cierre al vacío (VAC), ofrece efectos favorables adicionales en la función mitocondrial y/o marcadores de estrés oxidativo en PAS.
Métodos: mediante un estudio observacional prospectivo, se incluyeron pacientes con PAS y APACHE II > 8 sin respuesta satisfactoria al manejo en la Unidad de Cuidados Intensivos. Dieciséis pacientes se sometieron a necrosectomía y 24 a necrosectomía + VAC cada 48 h. Se dividieron en sobrevivientes y fallecidos. Se determinó la fluidez de la membrana submitocondrial de las plaquetas y la hidrólisis de la F0F1-ATPasa como función mitocondrial. El estrés oxidativo/nitrosativo se midió mediante lipoperóxidos (LPO), óxido nítrico (ON), fluidez de la membrana de eritrocitos y capacidad antioxidante total (CAT).
Resultados: la fluidez de membrana de partículas submitocondriales de plaquetas se mantuvo incrementada significativamente durante todo el estudio y aumentó al final en los fallecidos tratados con necrosectomía + VAC vs. los sobrevivientes (p < 0,041). La hidrólisis se encontró significativamente elevada desde el inicio hasta el final en todos los pacientes, predominando en los que fallecieron tratados con necrosectomía (p < 0,03). Hubo aumento de LPO en todos los pacientes aunque la necrosectomía fue más eficaz en la disminución al final en sobrevivientes (p < 0,039). El ON se encontró incrementado durante el resultado basal-final en sobrevivientes y fallecidos en ambas alternativas de tratamiento. La fluidez de la membrana de eritrocitos se encontró incrementada en los sobrevivientes tratados con necrosectomía + VAC y se normalizó al final (p < 0,045). La CAT se encontró consumida en todos los pacientes durante todo el estudio.
Conclusiones: se encontró disfunción mitocondrial y estrés oxidativo/nitrosativo con consumo importante de los antioxidantes sistémicos. La necrosectomía fue más eficiente al eliminar mejor los LPO. La necrosectomía + VAC mejoró la fluidez de la membrana de eritrocitos e incrementó la sobrevida.
Palabras clave: Pancreatitis aguda. Disfunción mitocondrial. Estrés oxidativo. Pancreatitis aguda severa.
Introduction
Severe acute pancreatitis (SAP) is defined as an inflammatory pancreatic condition with peripancreatic and multiple organ involvement that may result in multiple organ dysfunction syndrome, with a mortality rate of 20-60 % (1). According to the Atlanta Classification, SAP is associated with local and/or systemic complications that may be present since acute pancreatitis (AP) early onset (2). The presence of acute respiratory, cardiovascular, and renal failure, as well as gastrointestinal bleeding, may predict a fatal outcome for SAP (3). Pancreatic enzymes become activated inside this organ and induce self-digestion, which together with inflammation triggers oxidative stress. Pancreatic enzymes may reach the bloodstream and stimulate inflammatory cytokine production by leukocytes (4), hence giving rise to both pancreatic and systemic complications (5,6). During the inflammation process oxidative stress plays a positive role by inducing cell proliferation, gene activation, and apoptosis. However, it is unclear when these actions become deleterious (7-9). Reactive oxygen species (ROS) may be closely related to pancreas inflammation, and condition AP severity. In SAP there is an imbalance between oxidant and antioxidant systems, with lipoperoxidation by product (LPO) overproduction. These substances may damage cell lipid membranes, proteins, carbohydrates, and nucleic acids (10), which makes of LPOs a significant factor when it comes to predict disease severity. Microcirculation dysfunction in SAP may result from impaired nitric oxide (NO) production as pathogenic factor (11). NO plays an oxidant role by triggering nitrosative stress, and an antioxidant role by protecting cells from oxidative stress (12-15). Total antioxidant capacity (TAC) provides a complete description of cell antioxidant system functioning, as it reflects the body's ability to prevent ROS-induced damage. Systemic antioxidant measurement may provide a wider view of SAP-related pathophysiologic changes, thus allowing consideration of different therapeutic alternatives (16). Abnormal erythrocyte membrane fluidity is thought to condition the hemorrheologic changes associated with AP pathogenesis (17).
Mitochondria are a cell's primary source of energy as they synthesize ATP for active ion transport and sustain membrane potential. Mitochondrial function and defective cell membranes may be involved in the pathogenesis of SAP by impairing energy metabolism and interfering in ATP production as a result of their inducing antigenic changes in cells by reducing oxygen, glucose, and inorganic phosphate consumption (18). Adequate fluidity in submitochondrial particles of platelets depends on cell environmental temperature and microviscosity. Fluidity of submicromolar forms may be measured using the excimer/monomer intensity ratio (Ie/Im) as previously reported (19). The F0F1-ATPase enzyme has a transmembrane portion (F0) that pumps protons across the membrane, and an extramembrane portion (F1), where ATP synthesis or hydrolysis occurs. The F0F1-ATPase complex alternatively functions as a synthase or hydrolase (uses or pumps protons). The synthetic function is the enzyme's primary role. Under pathologic conditions synthesis decreases and hydrolysis increases, which results in enhanced energy catabolism (20).
Several multifactorial scoring systems are available that incorporate clinical and biochemical criteria to assess SAP, including among others Ranson criteria, the Glasgow scale, and the APACHE II (Acute Physiology and Chronic Health Evaluation II) classification. The sensitivity and specificity of these systems to predict pancreatitis severity is 55-90 %. The APACHE-II classification was not specifically developed to assess SAP but has proven to be an early reliable tool (21).
Treatment alternatives for SAP include necrosectomy, a surgical procedure of choice for patients with infected pancreas necrosis and multiple organ failure (22). The vacuum-assisted negative pressure system (VAC) is a modern therapeutic modality for the management of infected wounds (23), with no reported additional benefits for SAP. Given that no surgical technique has been as yet described as most appropriate for the management of pancreatic necrosis, we set out to assess whether necrosectomy alone or plus VAC offers any additional benefit in terms of mitochondrial function and/or oxidative stress markers in SAP.
Patients and methods
Using a prospective observational study design 40 patients diagnosed with SAP and admitted to the intensive care unit (ICU) at Hospital de Especialidades, Centro Médico Nacional de Occidente, Instituto Mexicano del Seguro Social with APACHE II > 8 and insufficient response to routine management were included. All of them required surgery and underwent open necrosectomy or necrosectomy plus VAC every 48 h. We expected VAC to improve clinical and chemical outcomes (24,25). The study's primary endpoint was mitochondrial function, and secondary endpoints included oxidative stress markers.
Clinical manifestations
Although the APACHE II classification is considered useful up to 48 h after hospital admission, in our study it was ongoing until patient discharge. Laboratory parameters measured included amylase, lipase, lactic dehydrogenase (LDH), alkaline phosphatase (AP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin total (TB), direct bilirubin (DB), prothrombin time (PT), platelets, complete blood count (CBC), haematocrit (Ht), leukocytes, glucose, urea, creatinine, sodium, potassium, and calcium. Rate assessments included stay in ICU, total hospital stay, complications, and mortality. Results from survivors and from deceased patients were analysed separately.
Blood was obtained as follows: 5 mL in a dry tube and 5 mL in a tube with 0.1 % ethylene-diamine-tetraacetic acide (EDTA). Plasma and serum were separated by centrifugation at 3,000 rpm for 10 min. Blood samples were drawn just before anesthetic induction for each surgery. Multiple samples were obtained during hospital stay but only the first one was considered as baseline and the last one as endpoint.
Membrane fluidity of erythrocytes and in submitochondrial particles of platelets
A platelet-rich plasma and erythrocytes were separated from the blood sample by centrifugation for 10 min at 7,000 x g. The supernatant was disposed of and the pellet was suspended in 200 mL of cold buffer (NaCl 140 mM, KCl 4.7 mM, MgCl 1.2 mM, KH2PO4 1.2 mM, dextrosa 11 mM, and HEPES 15 mM). White ghosts and platelets were stored (70 µL) at -80 oC until processing according to the method by Baracca et al. (20). The measurement of membrane fluidity in erythrocytes and in submitochondrial particles of platelets was performed by adding fluorescent spectroscopic ethanol (DPP) to the buffer: 10 mM Tris-HCl (pH 7.8) and 0.2 mM of DPP were diluted and mixed with membranes in a 1:1500 molar reaction (membrane phospholipids fluorescence). Alternatively, 0.25 mg of submitochondrial protein and 0.1 nmol of DPP were diluted in buffer. Mixtures were incubated in the dark at 4 oC for 3 h to ensure maximum DPP incorporation to membranes. Fluorescence was measured at 24 oC with a spectrometer (Perkin Elmer, LS50B). The fluorophore was excited at 329 nm, and the fluorescent intensity ratio (Ie/Im) at 378 and 476 nm (19).
F0F1-ATPase activity
Using phosphate-free test tubes 30 µL of sample (mitochondria) and 20 µL of ATP (100 mM) were addes to 1 mL of HEPES buffer (125 mM KCl, 40 mM Mops [pH 8], 3 mM MgCl2). Tubes were shaken in a vortex mixer and then placed in a bain-marie container for 10 min at 40 oC to facilitate the reaction. The reaction was then halted with 200 µL of 30 % trichloroacetic acid every 15 seconds between samples. The tubes were centrifuged at 3,500 rpm for 10 min. Then, 800 µL of supernatant were withdrawn, and 1 mL of 3.3 % ammonium molybdate and 100 µL of 10 % ferrous sulphate were added. The tubes were left to rest for 20 min at room temperature, and readings were performed at a wavelength of 660 nm.
Oxidative stress markers
Malondialdehyde (MDA) and 4-hydroxialkenes (4-OHA) were measured to reflect LPOs. The assay is based on the reaction of chromogen N-methyl-2-phenylindole (R1) with MDA and 4-OHA at 45 oC. The instructions issued by the manufacturer were followed (Oxford Biomedical Research, Inc., FR12); 140 µL of serum were placed in Eppendorf 1 mL tubes, 455 µL of reagent R1 were added, and the mix was vortex shaken. Then 105 µL of methanesulfonic acid (reagent R2) were added and shaken. Samples were incubated for 1 h at 45 oC and centrifuged at 15,000 x g for 10 min to obtain the supernatant; 150 µL of supernatant were transferred to a plate. Absorbance was read at a wavelength of 586 nm.
Nitric oxide
Samples were deproteinized by adding 6 mg of zinc sulfate at 400 µL of sample, followed by centrifugation at 10,000 x g and 4 oC for 10 min. The supernatant was withdrawn and stored at -80 oC (26). Following manufacturer guidelines colorimetry ensued (Nitric Oxide Assay Kit, protocol 482650, Calbiochem®), and NO metabolites (nitrites/nitrates) were measured. Then 85 µL of standard or sample, 10 µL of nitrate reductase, and 10 µL of 2 mM NADH were added. The plate was shaken for 20 min at room temperature. Then 50 µL of R1 and 50 µL of R2 were added. The sample was vortex shaken for 5 min at room temperature. The plate was read at 540 nm.
Total antioxidant capacity
Measurements were performed using the colorimetric kit (Total Antioxidant Power Kit, TA02.090130, Oxford Biomedical Research®); to obtain concentration in mM of uric acid equivalents, samples and standards were diluted to 1:40, and 200 µL of reagent were added to each well. The plate was read at 450 nm as reference. Subsequently 50 µL of copper solution were added to each well, and the whole was then incubated for 3 min at room temperature. Then 50 µL of stop solution were added, and the plate was read at 450 nm. The results of both readings were subtracted to obtain concentration. The final result was multiplied by the dilution factor.
Ethical considerations
The study was approved by the local ethics committee with number R-2009-1301-86. Identification codes were assigned to ensure patient confidentiality. An informed consent was signed by patients or family members before the study according to both national and international laws. Good clinical practice recommendations and Helsinki Declaration principles as updated in 1975 (1983 revision) were complied with.
Statistical analysis
This was performed using the SPSS version 21 software. Quantitative variables were expressed as mean ± standard error values, and qualitative variables as frequencies and percentages. Data followed a normal distribution (Shapiro-Wilk). Between-groups comparisons were analyzed using the t-test for independent samples; the t-test for related samples was used for the intra-group analysis of the baseline-endpoint result. Values were considered significant with a two-tailed p < 0.05.
Results
Forty consecutive patients were recruited from February 2009 to February 2013. In all, 22 males and 18 females with 35-55 yrs of age were included. Stay in the ICU was 18-25 days, and total hospital stay was 30-60 days. Sixteen patients were always managed with open necrosectomy every 48 h. Twenty-four additional patients always underwent necrosectomy + VAC every 48 h. Patients managed with necrosectomy + VAC were younger, and those who died had longer ICU stays (p < 0.0086). Mortality was higher among those receiving necrosectomy (62.5 %) and lower among those managed with necrosectomy + VAC (29.1 %). A blood sample was drawn from 24 age- and gender-matched healthy volunteers to establish normal values. Patients with mild AP were not included.
Table I lists altered baseline levels for amylase, lipase, ALT, AST, TB, DB, and AP. Final results for the following parameters are significantly improved: Amylase, lipase, LDH, AST, AP, and potassium. However, the final improvement of lab parameters had no impact on patient outcome; even prothrombin time was lengthened in patients who died.

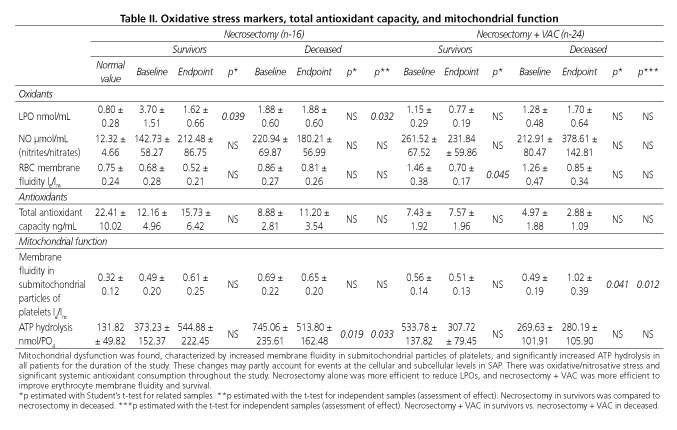
Mitochondrial function and oxidative stress markers are listed in table II.
Fluidity in submitochondrial particles of platelets
The normal value for submitochondrial particles in platelets was 0.32 ± 0.12 le/lm. The baseline value for survivors managed with necrosectomy increased to 0.49 ± 0.20 le/lm and then eventually to 0.61 ± 0.25 le/lm. In those managed with necrosectomy who died the result remained unchanged from a baseline value of 0.69 ± 0.22, with a final 0.65 ± 0.20 le/lm. There was also an increase in fluidity in submitochondrial particles of platelets among survivors managed with necrosectomy + VAC from start to end, with values of 0.56 ± 0.14 and 0.51 ± 0.13 le/lm, respectively. Patients who died after necrosectomy + VAC also had an increase from an initial 0.49 ± 0.19 le/lm, with a final value of 1.02 ± 0.39 le/lm (p < 0.04 and p < 0.12). The analysis of those subjected to necrosectomy vs. those who died after necrosectomy + VAC found a higher increase in fluidity in the latter (p < 0.017).
F0F1-ATPase hydrolysis activity
Normal ATP hydrolysis was 131.82 ± 49.82 nmol/PO4 (mg of protein). Survivors managed with necrosectomy showed a significant increase in baseline enzyme activity, 373.23 ± 152.37 nmol/PO4, which finally increased to 544.88 ± 222.45 nmol/PO4 vs. normal (p < 0.0001), with no difference between baseline and endpoint values. In those who failed to survive after necrosectomy, initial hydrolysis showed a peak value of 745.06 ± 235.61 nmol/PO4, with persistence until a final 533.78 ± 187.32 nmol/PO4 (p < 0.0001 vs. normal) and in baseline-final value (p < 0.019). The final result for ATP hydrolysis among survivors managed with necrosectomy + VAC was found to be significantly increased, 533.78 ± 137.82 nmol/PO4, vs. normal (p < 0.0001), with a tendency to lower final values in survivors managed with necrosectomy + VAC, although increased enzyme activity persisted at 307.72 ± 79.45 nmol/PO4. Among the deceased there was no change between baseline -269.63 ± 101.91 nmol/PO4- and final -280.19 ± 105.90 nmol/PO4- levels. The analysis of the established surgical procedure's effect shows a significant difference in enzyme levels between survivors and deceased patients managed only with necrosectomy (p < 0.033). When comparing the effect of necrosectomy vs. necrosectomy + VAC among the deceased, the former technique alone was more efficient in partially reducing enzyme levels (p < 0.03).
Lipoperoxidation by products
The normal value was 0.80 ± 0.28 nmol/mL, with a significant increase in survivors managed with necrosectomy; baseline value of 3.70±1.51 nmol/mL vs. normal (p < 0.05), with a final decrease to near normal levels, 1.62 ± 0.66 nmol/mL (p < 0.039). Those who died after necrosectomy showed no changes between baseline -1.88 ± 0.60 nmol/mL- and endpoint -1.53 ± 0.48 nmol/mL- results. The baseline value for survivor's managed with necrosectomy + VAC was 1.15 ± 0.29 nmol/mL, with a final decrease to normal levels, 0.77 ± 0.20 nmol/mL. LPOs were found to be increased at baseline for those who died -1.28 ± 0.48 nmol/mL- with persistence in endpoint results -1.70 ± 0.64 nmol/mL. In the analysis of those who survived, necrosectomy was more efficient to reduce LPOs vs. necrosectomy + VAC (p < 0.006). When assessing effect, necrosectomy alone was more efficient in reducing LPO levels as compared to necrosectomy + VAC (p < 0.035).
Nitrites/nitrates
The normal value was 12.32 ± 4.66 µmol/mL. There was a significant increase at baseline -142.73 ± 58.27 µmol/mL- vs. normal (p < 0.034) among those treated with necrosectomy, with a final increase to 212.48 ± 86.75 µmol/mL and no difference in baseline-endpoint results.
Those who died after necrosectomy also showed an increased from baseline -220.94 ± 69.87 µmol/mL- to endpoint -180.21 ± 56.99 µmol/mL- vs. normal (p < 0.026), with no significant difference between baseline and endpoint. Survivors managed with necrosectomy + VAC, also showed increased levels at baseline -261.52 ± 67.52 µmol/mL- and endpoint -231.84 ± 59.86 µmol/mL- vs. normal (p < 0.029). Those who died had increased nitrite/nitrate levels from baseline -212.91 ± 80.47 µmol/mL- with a final peak -378.61 ± 142.81 µmol/mL- (p < 0.025) vs. normal. No differences were seen between baseline and final values.
Membrane fluidity of erythrocytes
Normal membrane fluidity in erythrocytes was 0.75 ± 0.24 Ie/Im. At baseline among survivors managed with necrosectomy + VAC it was increased -1.46 ± 0.38Ie/Im (p < 0.045)- vs. normal, and the parameter was eventually returned to normal -0.70 ± 0.17Ie/Im (p < 0.045 for baseline- endpoint). At baseline, those who died had 1.26 ± 0.47Ie/Im, with a final value of 0.85 ± 0.34Ie/Im. In contrast, those who underwent necrosectomy and then survived showed reduced membrane fluidity in erythrocytes at baseline -0.68 ± 0.28Ie/Im- and at endpoint -0.52 ± 0.21Ie/Im; subjects who died kept unchanged baseline and endpoint values: 0.86 ± 0.27Ie/Im and 0.81 ± 0.26Ie/Im, respectively.
Total antioxidant capacity
Normal TAC was 22.41 ± 10.02 ng/mL. Baseline results from survivors managed with necrosectomy + VAC showed significantly reduced levels -7.43 ± 1.92 ng/mL- vs. normal (p < 0.0016), with no improvement seen in the final result -7.57 ± 1.96 ng/mL (p < 0.004). Baseline levels for those who passed away after necrosectomy + VAC were found to be even lower -4.97 ± 1.88 ng/mL (p < 0.0007)- and minimal levels were found at endpoint -2.88 ± 1.09 ng/mL (p < 0.0001 vs. normal). Baseline results among survivors after necrosectomy was 12.16 ± 4.96 ng/mL, with a slight final improvement at endpoint -15.73 ± 6.42 ng/mL. Baseline value among the deceased after necrosectomy was 8.88 ± 2.81, and final value was 11.20 ± 3.54 ng/mL.
Discussion
While the group undergoing necrosectomy + VAC was younger by a decade, age had no significant impact on patient survival. However, those who died after necrosectomy + VAC had a significantly longer ICU stay, which is in contrast with the better survival of those who received this surgical procedure. This fact alone may account for prolonged ICU stay since continued systematic management significantly improves survival for patients with SAP (27). Regarding amylase and lipase laboratory measurements, these were not useful to differentiate AP from SAP, or to predict pancreatitis progression: these results could not foretell final outcomes (28).
Membrane fluidity in submitochondrial particles of platelets was found to be increased for all patients in the study. This increase was greater for the final-baseline results of those who failed to survive with necrosectomy + VAC (p < 0.012), which translates into severe impairment of the inner membrane in submitochondrial particles of platelets, which in turn impairs oxidative phosphorylation and the production of enzymes involved in metabolite transport and usage, as well as in ATP production by cells. Increased membrane fluidity in the submitochondrial particles of platelets is suggestive of a high responsiveness of said inner membrane to oxidative stress, especially to increased LPOs, which may facilitate cell death by necrosis or apoptosis as previously reported for liver cholestasis (29). Our results are in strong contrast with those reported by Ortiz et al. (19), which show decreased membrane fluidity in submitochondrial particles of platelets in Alzheimer's disease, typically a chronic condition with a variable course. However, SAP is an active disease, similar to septic conditions (30).
Normal F1F0-ATPase hydrolytic activity remains stable and is closely related to synthesis. In the present study enzyme activity was characterized by significant increases throughout the research, both among survivors and the deceased, with a baseline peak in the patients who died after necrosectomy + VAC. The above suggests severe dysregulation of the energy production/consumption ratio by cells in SAP, which translates into significant energy catabolism in this condition, as previously reported for Alzheimer's disease (31).
We found oxidative stress characterized by a significant increase in LPOs. Necrosectomy was considered more efficient for LPO clearance between baseline and endpoint in survivors, though. MDA+4-OHA ROS can oxidize multiple lipid and low-density lipoprotein molecules, and condition that pancreatic proteases play a role in xanthine dehydrogenase cleavage to xanthine oxidase, thus giving rise to hypoxanthine (ATP degradation by product), xanthine radicals and superoxide anions, which damage cell membrane-related phospholipids and play an active role in triggering systemic inflammatory response syndrome and inflammatory cytokine activation (32). In some AP reports glutathione and other sulfhydryl compounds are found to be depleted, with increased LPOs and a higher utilization of both enzymatic and non-enzymatic antioxidants (33). In our study a significant reduction in body antioxidant capacity was seen both in survivors and non-survivors throughout the follow-up period, which supports the presence of oxidant/antioxidant imbalance. Oxidative stress may also be estimated from increased superoxide anions, hydrogen peroxide, hydroxyl radicals, and reactive nitrogen species from NO. NO metabolites increased from baseline in the present study, hence the presence of nitrosative stress may be considered. When NO levels are higher than required the guanylate cyclase enzyme becomes activated, glycolysis is inhibited, ATP production decreases in the mitochondrial respiratory chain, DNA replication is also inhibited, and NO reacts with superoxide anions to form peroxynitrite (34).
The impaired physical properties of cell membranes by LPOs may lead to defects strongly associated with systemic disease, as they alter the normal structure of plasma membranes in erythrocytes, thus inducing functional changes in: a) Enzyme activity; b) ion and other substances transportation; c) osmotic stability; d) oxygen diffusion; and e) membrane receptor activity, where reduced or increased erythrocyte viscoelasticity results in impaired blood flow and tissue perfusion (35). We found no reports on erythrocyte fluidity in AP, but erythrocyte membrane rigidity has been reported in subjects with essential hypertension as compared to non-hypertensive subjects (36). In this study, erythrocyte membrane fluidity behaved irregularly between both surgical management approaches and between survivors and non-survivors. In addition to facilitating deformation, this irregular fluidity may result in impaired systemic microcirculation by inducing both structural and functional changes in relation to LPOs (37).
The importance of the physiological relationship between nitrosative stress, systemic antioxidant consumption, and mitochondrial dysfunction as exhibited by patients managed with necrosectomy alone resulted in a higher mortality rate (62.5 %) as compared to the available literature (7-42 %) (38). Patients treated by adding the VAC system had a lower mortality (29.1 %) but membrane fluidity in submitochondrial particles of platelets increased. Hence we consider that the use of a VAC system may confer benefits regarding mortality. However, the use of necrosectomy alone offers the additional benefit of significantly reduced LPOs.
Baseline APACHE II scores increased in patients who eventually died. We consider that some APACHE II items and the VAC system predict AP severity more accurately than isolated demographic variables such as age (39). The benefit of VAC seemingly results from its facilitating the clearance of exudates rich in inflammatory substances, and its use may have a positive influence for patients with SAP.
It seems that regular, systematic necrosectomy, whether alone or plus VAC, is insufficient to manage the abdominal catastrophe of SAP. The presence of oxidative/nitrosative stress, and systemic antioxidant consumption in SAP are associated with a poor prognosis. The measurement of oxidative stress markers, antioxidant status, and mitochondrial function during the course of AP may provide a wider view of the pathophysiological changes that occur prior to AP progression to SAP, and help consider other therapy options with a potential to reduce both morbidity and mortality.
References
1. Al Mofleh IA. Severe acute pancreatitis: Pathogenetic aspects and prognostic factors. World J Gastroenterol 2008;14:675-84. [ Links ]
2. Tao H-Q, Zhang J-X, Zou S-C. Clinical characteristics and management of patients with early acute severe pancreatitis: Experience from a medical center in China. World J Gastroenterol 2004;10:919-21. [ Links ]
3. Kong L, Santiago N, Han T-Q, Zhang S-D. Clinical characteristics and prognostic factors of severe acute pancreatitis. World J Gastroenterol 2004;10:3336-8. [ Links ]
4. Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc 2002;56(6):S226-30. [ Links ]
5. Banks PA, Freeman ML. Practice Parameters Committee of the American College of Gastroenterology Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379-400. [ Links ]
6. Gloor B, Müller CA, Worni M, Martignoni ME, Uhl W, Büchler MW. Late mortality in patients with severe acute pancreatitis. Br J Surg 2001;88:975-9. [ Links ]
7. Sanfey H, Bulkley GB, Cameron JL. The role of oxygen free radicals in the pathogenesis of acute pancreatitis. Ann Surg 1984;200:405-12. [ Links ]
8. Thareja S, Bhardwaj P, Sateesh J, Saraya A. Variations in the levels of oxidative stress and antioxidants during early acute pancreatitis. Trop Gastroenterol 2009;30:26-31. [ Links ]
9. Galley HF, Davies MJ, Webster NR. Xanthine oxidase activity and free radical generation in patients with sepsis syndrome. Crit Care Med 1996;24:1649-53. [ Links ]
10. Park BK, Chung JB, Lee JH, Suh JH, Park SW, Song SY, et al. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol 2003;9:2266-9. [ Links ]
11. Kleinhans H, Mann O, Schurr PG, Kaifi JT, Hansen B, Izbicki JR, et al. http://www.ncbi.nlm.nih.gov/pubmed?term=Strate%20T%5BAuthor%5D&cauthor=true&cauthor_uid=16718818. Oxygen radical formation does not have an impact in the treatment of severe acute experimental pancreatitis using free cellular hemoglobin. World J Gastroenterol 2006;12:2914-8. [ Links ]
12. Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H. Protective role of endothelial nitric oxide synthase. J Pathol 2003;199:8-17. [ Links ]
13. Mohanakumar KP, Thomas B, Sharma SM, Muralikrishnan D, Chowdhury R, Chiueh CC. Nitric oxide: An antioxidant and neuroprotector. Ann N Y Acad Sci 2002;962:389-401. [ Links ]
14. Erusalimsky DJ, Moncada S. Nitric oxide and mitochondrial signaling from physiology to pathophysiology. ATVB 2007;27:2524-31. [ Links ]
15. Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem 1998;273:14085-9. [ Links ]
16. Roth E, Manhart N, Wessner B. Assessing the antioxidative status in critically ill patients. Curr Opin Clin Nutr Metab Care 2004;7:161-8. [ Links ]
17. Zhao T, Guo J, Li H, Huang W, Xian X, Ross CJ, et al. Hemorheological abnormalities in lipoprotein lipase deficient mice with severe hypertriglyceridemia. Biochem Biophys Res Commun 2006;341:1066-71. [ Links ]
18. Fukuyama H, Ogawa M, Yamauchi H, Yamauchi S, Kimura J, Yonokura Y, et al. Altered cerebral energy metabolism in alzheimer's disease: A PET study. J Nucl Med 1994;35:1-6. [ Links ]
19. Ortiz GG, Pacheco-Moisés F, Hafidi ME, Jiménez-Delgado A, Macías-Islas MA, Rosales-Corral SA, et al. Detection of membrane fluidity in submitochondrial particles of platelets and erythrocyte membranes from Mexican patients with Alzheimer disease by intramolecular excimer formation of 1,3 dipyrenylpropane. Disease Markers 2008;24:151-6. [ Links ]
20. Baracca A, Barogi S, Carelli V, Lenaz G, Solaini G. Catalytic activities of mitocondrial ATP synthase in patients with mitocondrial DNAT8993G mutation in the ATPase 6 gene encoding subunit a. J Biol Chem 2000;275:4177-82. [ Links ]
21. Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, et al. http://www.ncbi.nlm.nih.gov/pubmed?term=Dixit%20VK%5BAuthor%5D&cauthor=true&cauthor_uid=24204087. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg 2013;2013:367581. [ Links ]
22. Vasiliadis K, Papavasiliou C, Al Nimer A, Lamprou N, Makridis C. The role of open necrosectomy in the current management of acute necrotizing pancreatitis: A review article. ISRN Surg 2013;2013:579435. [ Links ]
23. Marinis A, Voultsos M, Grivas P, Dikeakos P, Liarmakopoulos E, Paschalidis N, et al. http://www.ncbi.nlm.nih.gov/pubmed?term=Rizos%20S%5BAuthor%5D&cauthor=true&cauthor_uid=24335462. Vacuum-assisted therapy accelerates wound healing in necrotizing soft tissue infections: Our experience in two intravenous drug abuse patients. Infez Med 2013;21:305-11. [ Links ]
24. Zagli G, Cianchi G, Degl'innocenti S, Parodo J, Bonetti L, Prosperi P, et al. http://www.ncbi.nlm.nih.gov/pubmed?term=Peris%20A%5BAuthor%5D&cauthor=true&cauthor_uid=22606389. Treatment of fournier's gangrene with combination of vacuum-assisted closure therapy, hyperbaric oxygen therapy, and protective colostomy. Case Rep Anesthesiol 2011;2011:430983. [ Links ]
25. Wondberg D, Larusson HJ, Metzger U, Platz A, Zingg U. Treatment of the open abdomen with the commercially available vacuum-assisted closure system in patients with abdominal sepsis. World J Surg 2008;32:2724-9. [ Links ]
26. Ghasemi A, Hedayati M, Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. J Med Sci Res 2007;2:29-32. [ Links ]
27. Pavlidis P1, Crichton S, Lemmich SJ, Morrison D, Atkinson S, Wyncoll D, et al. Improved outcome of severe acute pancreatitis in the intensive care unit. Crit Care Res Pract 2013;2013:897107. [ Links ]
28. Devanath A, Kumari J, Joe J, Peter S, Rajan S, Sabu L, et al. Usefulness of lipase / amylase ratio in acute pancreatitis in south Indian population. Indian J Clin Bioquem 2009;24:361-5. [ Links ]
29. Tiao MM, Lin TK, Wang PW, Chen JB, Liou CW. The role of mitochondria in cholestatic liver injury. Chang Gung Med J 2009;32:346-53. [ Links ]
30. Leverve XM. Mitochondrial function and substrate availability. Crit Care Med 2007;35:S454-60. [ Links ]
31. Bosetti F1, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging 2002;23:371-6. [ Links ]
32. Abu-Hilal M, McPhail MJ, Marchand L, Johnson CD. Malondialdehyde and superoxide dismutase as potential markers of severity in acute pancreatitis. JOP 2006;7:185-92. [ Links ]
33. Bansal D, Bhalla A, Bhasin DK, Pandhi P, Sharma N, Rana S, et al. http://www.ncbi.nlm.nih.gov/pubmed?term=Malhotra%20S%5BAuthor%5D&cauthor=true&cauthor_uid=21546719. Safety and efficacy of vitamin-based antioxidant therapy in patients with severe acute pancreatitis: A randomized controlled trial. Saudi J Gastroenterol 2011;17:174-9. [ Links ]
34. Carrizo PH, Dubin M, Stoppani AO. Physiopathologic effects of nitric oxide and their relationship with oxidative stress. Medicina (B Aires) 1998;58:367-73. [ Links ]
35. Kowalczyk E, Kowalski J, B?aszczyk J, Gwodziski L, Ciewierz J, Sienkiewicz M. Estimation of cell membrane properties and erythrocyte red-ox balance in patients with metabolic syndrome. Mol Biol Rep 2012;39:11113-8. [ Links ]
36. Tsuda K, Nishio I. Membrane fluidity and hypertension. Am J Hypertens 2003;16: 259-61. [ Links ]
37. Zicha J, Kunes J, Devynck MA. Abnormalities of membrane function and lipid metabolism in hypertension: A review. Am J Hypertens 1999;12:315-31. [ Links ]
38. Wang X, Cui Z, Li H, Saleen AF, Zhang D, Miao B, et al. Nosocomial mortality and early prediction of patients with severe acute pancreatitis. J Gastroenterol Hepatol 2010;25:1386-3. [ Links ]
39. Pearce CB, Gunn SR, Ahmed A, Johnson CD. Machine learning can improve prediction of severity in acute pancreatitis using admission values of APACHE II score and C-reactive protein. Pancreatology 2006;6(1-1):123-31. [ Links ]
![]() Correspondence:
Correspondence:
Alejandra Guillermina Miranda-Díaz
Centro Universitario de Ciencias de la Salud
Universidad de Guadalajara, Guadalajara
Jalisco. Mexico
e-mail: kindalex1@outlook.com
Received: 31-10-2013
Accepted: 18-09-2014











 texto en
texto en