My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.3 Madrid Mar. 2015
Risk of bleeding in patients undergoing percutaneous endoscopic gastrotrostomy (PEG) tube insertion under antiplatelet therapy: A systematic review with a meta-analysis
Riesgo de sangrado en pacientes sometidos a inserción de un tubo de gastrostomía percutánea endoscópica y en tratamiento antiplaquetario: una revisión sistemática con metaanálisis
Alfredo J. Lucendo1, Tomás Sánchez-Casanueva2, Olga Redondo3, José M. Tenias4 and Ángel Arias3
Departments of 1Gastroenterology and 2Hospital Pharmacy. Hospital General de Tomelloso. Tomelloso, Ciudad Real, Spain.
3Research Unit. Complejo Hospitalario La Mancha Centro. Alcázar de San Juan, Ciudad Real. Spain.
4Unitat Docent de Medicina Familiar i Comunitària. Escola Valenciana d' Studis de la Salut. Valencia, Spain
ABSTRACT
Background and aim: Patients undergoing percutaneous endoscopic gastrostomy (PEG) tube placement often are under antiplatelet therapy with a potential thromboembolic risk if these medications are discontinued. This systematic review aims to assess if maintaining aspirin and/or clopidogrel treatment increases the risk of bleeding following PEG placement.
Methods: A systematic search of the MEDLINE, EMBASE, and SCOPUS databases was developed for studies investigating the risk of bleeding in patients on antiplatelet therapy undergoing PEG tube insertion. Summary estimates, including 95 % confidence intervals (CI), were calculated. A fixed or random effects model was used depending on heterogeneity (I2). Publication bias risks were assessed by means of funnel plot analysis.
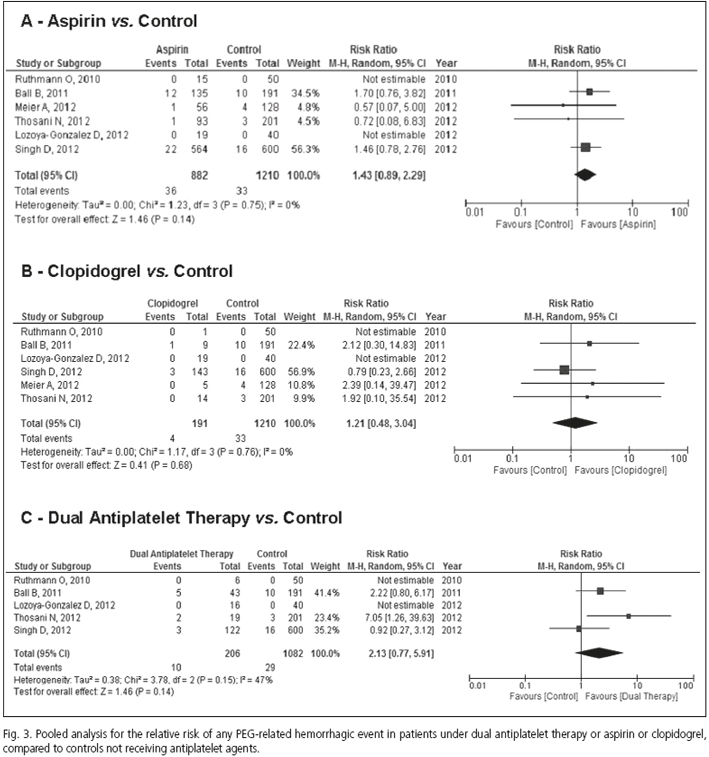
Results: Eleven studies with a total of 6,233 patients (among whom 3,665 were undergoing antiplatelet treatment), met the inclusion criteria and were included in the quantitative summary. Any PEG tube placement-related bleeding was found in 2.67 % (95 % CI 1.66 %, 3.91 %) of the entire population and in 2.7 % (95 % CI 1.5 %, 4.1 %) of patients not receiving antiplatelet therapy. Pooled relative risk (RR) for bleeding in patients under aspirin, when compared to controls, was 1.43 (95 % CI 0.89, 2.29; I2 = 0 %); pooled RR for clopidogrel was 1.21 (95 % CI 0.48, 3.04; I2 = 0 %) and for dual antiplatelet therapy, 2.13; (95 % CI 0.77, 5.91; I2 = 47 %). No significant publication bias was evident for the different medications analyzed.
Conclusion: Antiplatelet therapy was safe among patients undergoing PEG tube insertion. Future prospective and randomized studies with larger sample sizes are required to confirm the results of this study.
Key words: PEG. Gastrostomy. Tube feeding. Gastric feeding tubes. Antiplatelet drugs. Aspirin. Clopidogrel.
Introduction
Percutaneous endoscopic gastrostomies (PEG) are widely used to provide enteral nutritional support to patients who are unable to ingest solid or liquid foods, despite having preserved absorption and motility functions of the gastrointestinal tract. In these cases, PEG tube feeding serves as an alternative to artificial parenteral nutrition and especially to nasogastric tubes, for the administration of food directly into the stomach, providing patients with the most suitable and physiological feeding option (1).
PEG placement is an endoscopic technique consisting in the insertion of a flexible tube to create a temporary or permanent communication between the abdominal wall and the gastric cavity, ensuring the direct passing of food into the patient's digestive tract. Since Ponsky and Gauderer described this technique (2), PEG tubes have replaced other surgical (3) and radiological (4) gastrostomy techniques as the method of choice for long term feeding of patients with a high range of acute and chronic conditions, in different situations (5,6), both in hospital and at home (7). The technique has been associated with significantly less overall complication rates (8,9) and is recognized as a minimally invasive procedure that eliminates the need for general anesthesia and reduced use of instrumentation.
PEG is considered as an invasive interventional endoscopic procedure potentially associated with a high risk of bleeding, a complication that has been reported in approximately 2.5 % of procedures in the early literature (10,11). Therefore, guidelines of the British Society of Gastroenterology (BSG) (12) and the American Society of Gastrointestinal Endoscopy (ASGE) (13) recommend the cessation of antiplatelet treatment with clopidogrel or ticlopidine seven days prior to "high-risk" endoscopic procedures. With regard to aspirin and others non-steroideal anti-inflammatory drugs (NSAIDs) endoscopic procedures may be performed while the patient is receiving this medication in the absence of a pre-existing bleeding diathesis (13). However, the aforementioned guidelines are based on expert opinion and best clinical practice, since there are no available prospective randomized clinical trials (RCT) to support them.
Patients undergoing PEG are commonly treated with aspirin and/or other antithrombotic agents, which are frequently used for treating or preventing several cardio- and cerebrovascular diseases. Discontinuation of antiplatelet medication in such situations is associated with increased risk of thrombosis, with severe or fatal consequences (14-16). Clopidogrel cessation may also result in rebound platelet hyperactivity contributing to increased thromboembolic complication in this setting (17). A major dilemma concerning patients taking these medications includes the potential risk of bleeding as a result of endoscopic intervention and the risk of thromboembolic events when such medications are withheld. As a result, several authors have evaluated in recent large retrospective studies, whether there is an association between periprocedural use of aspirin, clopidogrel, or ticlopidine and bleeding in patients who underwent PEG tube placement without withdrawing this medication (18-22). According to these studies, the use of antiplatelet therapy was not a risk factor of post procedure bleeding in such patients.
However, the safety of using PEG tube placement alongside antiplatelet treatment has yet to be systematically analyzed in order to provide clinicians and endoscopists with useful evidence for decision making with regard to the complex management of the risk and benefits in these delicate situations.
This research aims to systematically review the evidence available on the safety of periprocedural treatment with antiplatelet drugs (specifically aspirin, clopidogrel, ticlopidine and dual antiplatelet therapy) in patients who underwent PEG tube placement, in terms of procedural-related bleeding complications, compared to the "standard" risk of PEG tube insertion in patients with no antiplatelet therapy, or where therapy was withheld.
Methods
This systematic review has been registered in the PROSPERO International prospective register of systematic reviews (www.crd.york.ac.uk/PROSPERO; register no. CRD42014013607), and was reported in accordance with the PRISMA statements (23,24).
Selection of studies
Source studies were identified by systematically searching 3 major bibliographic databases (PUBMED, EMBASE, and Scopus) for the period up to September 2014. To this end, a predetermined protocol was used in accordance with the quality of reporting meta-analyses of observational studies in epidemiology (23,24).
Comprehensive search criteria were used to identify articles dealing with risk of bleeding in patients receiving antiplatelet treatment and undergoing PEG tube placement. We consulted the thesauri for MEDLINE (MESH) and EMBASE (EMTREE) using the following search strategy: (gastrostomy[MeSH Terms] OR gastrostomy feeding OR endoscopic gastrostomy complications OR percutaneous gastrostomy OR gastrostomy tube OR endoscopic gastrostomy OR percutaneous endoscopic gastrostomy OR peg tube) AND ("Platelet Aggregation Inhibitors" [Pharmacological Action] OR bleeding OR Hemorrhage" [Mesh] OR hemorrhag* OR antiplatelet OR antithrombotic agents). For the Scopus database, only free text searches with truncations were carried out. The search was not restricted with regard to date or language of publication.
We also examined the reference lists from retrieved articles and abstracts of conference proceedings to identify relevant studies. Abstracts books of the annual Digestive Diseases Week, American College of Gastroenterology Meeting and the United European Gastroenterology Week for the period 2005 to 2014 (if available) were also examined. Four reviewers (AJL, TS-C, OR-G, & AA) independently screened the database search for titles and abstracts. If any of the reviewers felt that a title or abstract met the study eligibility criteria, the full text of the study was retrieved.
Inclusion criteria
1. RCTs, observational prospective and retrospective studies, and case series reports were included if data on early and/or late complications of PEG tube insertion in patients receiving antiplatelet therapy were provided. Publications were included irrespective of whether these were the main focus of the article.
2. Studies evaluating any kind of antiplatelet therapy, including aspirin at different dosages, clopidogrel, ticlopidine or dual antiplatelet therapy in patients undergoing PEG tube placement. Exposure to antiplatelet agents was defined as any intake of the drug within the 48 hours prior to PEG tube insertion or within the following 24 hours after PEG placement.
3. Studies providing objective quantitative data on the occurrence of any bleeding episodes after PEG tube placement under the aforementioned conditions.
Exclusion criteria
1. Studies reporting on non-endoscopic gastrostomy tube placement (radiological or surgical gastrostomies).
2. Studies reporting on patients under anticoagulant therapy (warfarin, low molecular weight heparins [LMWH]) or unfractionated heparins (UFH), along or together with antiplatelet drugs.
3. Review articles on the safety and efficacy of PEG tube feeding that did not provide original data on antiplatelet therapy; clinical guidelines and consensus documents.
4. Studies not carried out on humans.
5. Studies providing duplicated information (i.e. repeated abstracts presented at different congresses or abstracts published later as a full-paper).
6. Subsets of cases or controls from a previously published article by the same authors.
Quality assessment
Cohort studies, case series, and case reports were evaluated for quality only if the article described all patients, the type and doses of antiplatelet drugs they used, the occurrence of any bleeding episode, and any additional procedural-related complication. Likewise, antiplatelet treatment and bleeding episodes or rate had to be specifically stated in the text as well as the time frames and the clinic or clinics in which the study was carried out. Quality assessment was checked with a specific evaluation form for observational studies based on the STROBE statements (25).
A study was considered to be at low risk for bias if each of the bias items could be categorized as low risk. On the contrary, studies were judged to have a high risk of bias if even one of the items was deemed high risk. Three investigators (AA, AJL, TSC) independently gave each eligible study an overall rating of high, low, or unclear risk of bias, and if disagreements emerged, a third reviewer (OR-G) was consulted.
Data extraction
Three reviewers (AA, AJL, and TS-C) independently extracted relevant information from each eligible study using a standardized data extraction sheet and then proceeded to cross-check the results. The data thus extracted included the last name of the first author; publication year; origin of the research; type of antiplatelet drug assessed; age and gender of study participants; sample size; methodological design and study period, whenever possible. At the same time, data on the key outcome, including occurrence of PEG-related bleeding episodes, together with its severity and outcomes, were extracted from all included studies. Disagreements between reviewers regarding data extraction were resolved through discussion. The authors of the various studies were contacted by e-mail for additional information if necessary.
Statistical analysis
PEG tube placement-related bleeding risks in patients with and without active antiplatelet therapy, were summarized with the aid of a fixed or random effects meta-analysis weighted for the inverse variance following DerSimonian and Laird's method. Results of this meta-analysis were expressed as pooled Relative Risks (RRs) with 95 % confidence interval (CI) for dichotomous outcomes. A value of p < 0.05 was considered statistically significant.
Heterogeneity between studies was assessed by means of a chi-square test (Cochran Q statistic) and quantified with the I2 statistic. If p < 0.1 and/or I2 > 50 %, there was significant heterogeneity and a random effects model was used. Generally, I2 was used to evaluate the level of heterogeneity, assigning the categories low, moderate, and high to I2 values of 25 %, 50 %, and 75 %, respectively (26). Publication bias was evaluated with the aid of a funnel plot, the asymmetry of which was assessed through Begg-Mazumda's rank test (27), and the Harbord and Egger tests (28,29).
For the primary outcome, planned subgroup analyses were performed based on the types of antiplatelet agents used (aspirin, clopidogrel, ticlopidine), low vs. high doses (> 300 mg/day) of aspirin, and dual antiplatelet usage. A sensitivity analysis was performed with regard to quality (risk of bias) and type of document (full-length article vs. abstract presented at conference proceedings). All calculations were made with StatsDirect statistical software version 2.7.9 (StatsDirect Ltd, Cheshire, UK) and Review Manager v.5 (The Cochrane Collaboration).
Results
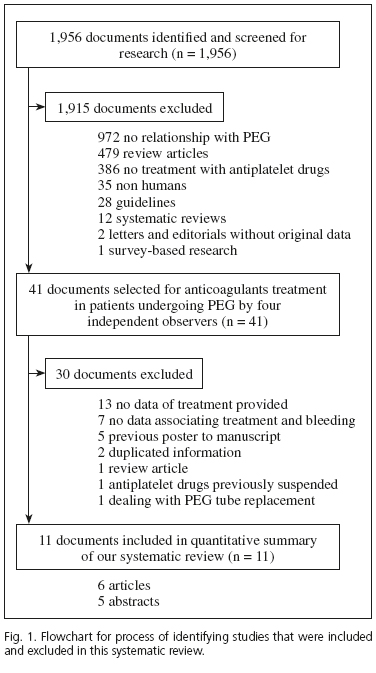
The search strategy identified 1,956 references to articles and abstracts; 1,915 documents were excluded after examining the title and abstract because they did not fulfill the inclusion criteria. The full text of the remaining 41 references that were considered to be potentially eligible was retrieved for detailed evaluation. Of these, 13 were excluded because they did not include data on treatment; 7 were not able to show the exact treatment administered to patients who bled; 5 abstracts were later published as full articles; 2 contained duplicated information reported at different meetings. Two further documents were respectively excluded due to being a review article and antiplatelet treatment being withdrawn prior to PEG insertion. One additional study dealing on PEG tube replacement was also excluded. Finally, 11 studies were included in the meta-analysis (Fig. 1).
Study characteristics
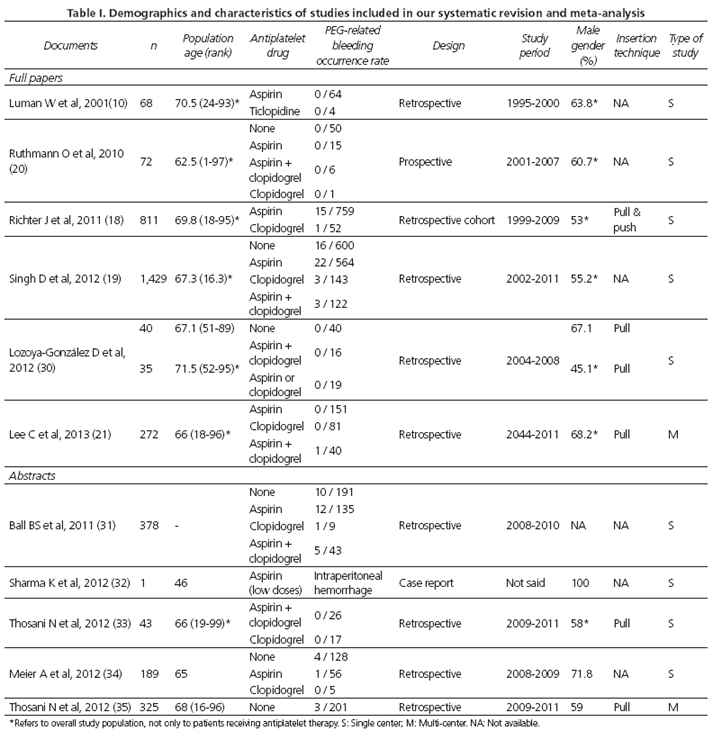
The major characteristics of each study are summarized in table I. Of the 11 documents, 6 were full text-articles and 5 were abstracts. Overall, data from 6,233 patients (among whom 3,665 were under antiplatelet therapy) undergoing PEG tube insertion were retrieved, with the size of the various study populations ranging from 1 to 1,625 cases in the largest series. The 11 documents included in this meta-analysis globally assessed 4 different antiplatelet agents: Aspirin; clopidogrel; both drugs combined in a dual antiplatelet therapy; and ticlopidine. It is worth noting that that all but one of the studies included patients undergoing PEG tube placement using the pull-through technique described by Ponsky and Gauderer (2).
Procedural-related risk of bleeding after PEG tube insertion
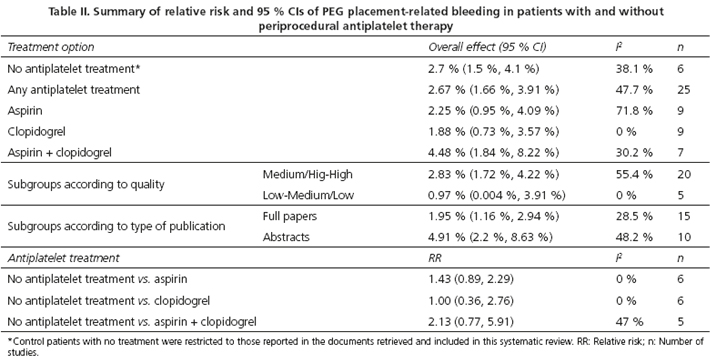
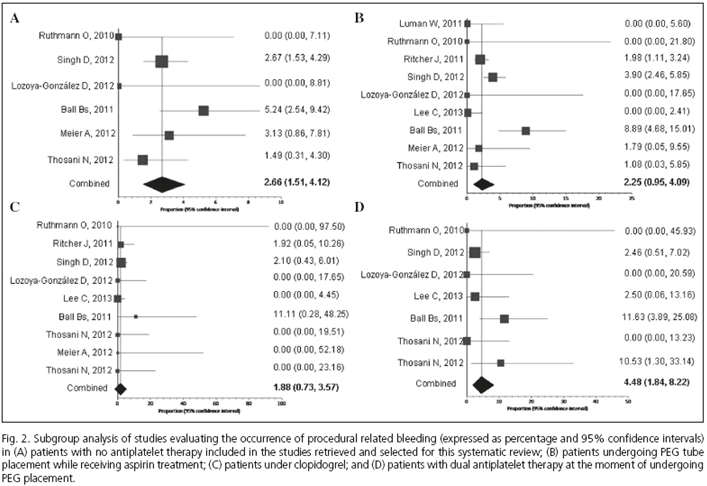
Any PEG tube placement related bleeding developed in 2.67 % (95 % CI 1.66, 3.91) of the entire population and occurred in 2.7 % (95 % CI 1.5, 4.1) of patients not receiving antiplatelet therapy in comparison to 2.25 % (95 % CI 0.95, 4.09) of those patients with aspirin; and 1.55 % (95 % CI 0.73, 3.57) of those receiving clopidogrel. The risk of bleeding in case of dual antiplatelet therapy overall resulted in 4.45 % (95 % CI 1.54, 5.22) (Table II and Fig. 2).
The use of ticlopidine was exclusively assessed in a single study (10), which included 4 patients who were receiving this treatment at the point of PEG tube insertion. Although no patient presented a procedure-related bleeding, limited data prevents us for developing further analysis.
Meta-analytical summaries on the risk of bleeding in patients under antiplatelet therapy
Aspirin and clopidogrel compared to control patients showed no increased risks of bleeding: Pool analysis of four out of the nine studies, which included patients undergoing PEG insertion under aspirin (19,31,33,34) and providing adequate information, showed that the RR of aspirin vs. control was 1.43 (95 % CI 0.89, 2.29; I2 = 0 %) (Fig. 3A). The RR for clopidogrel vs. control was 1.21 (95 % CI 0.48, 3.04; I2 = 0 %), according to pool analysis of treated patients (19,31,33,34) (Fig. 3B). No significant publication bias was documented according to funnel plot analysis and Egger's and Harbord-Egger's test (being p values for aspirin 0.134 and 0.158, respectively). In the case of clopidogrel, p values for Egger and Harbord-Egger tests were 0.083 and 0.946, respectively.
Three out of the seven different studies that assessed the use of dual antiplatelet therapy (19,31,33) provided adequate information to allow for the analysis of the risk of post-procedural PEG tube insertion bleeding. In pooled analysis of these patients (Fig. 3C), the RR for any hemorrhagic episode in patients under dual antiplatelet compared to control patients was 2.13 (95 % CI 0.77, 5.91), with a moderate heterogeneity among the studies included (I2 = 47 %). No significant publication bias was noted for documents reporting on dual antiplatelet use in patients undergoing PEG tube placement (p value for Egger test = 0.486; p value for Harbord-Egger test = 0.906).
Death related-bleeding
No bleeding-related mortality was reported in the retrieved documents. Less than half of the patients presented in the studies had clinically significant bleeding and underwent repeat endoscopy (19,21). The remaining cases were controlled by placing packing material around the wound site; applying pressure or a stitch to the bleeding site. Angiographic embolization was rarely needed (21). In fact, the use of antiplatelet agents was not identified as an independent risk factor related to mortality in the multivariate analysis of one research paper (36).
Subgroup analyses
Finally, an analysis of subgroups categorized according to quality and type of document, was carried out (Table II). Most of the selected studies were considered to be at least acceptable in quality, although the occurrence of bleeding was slightly higher in studies of high/high-mild quality compared with that found in low/low-mild quality studies (2.83 % vs. 0.97 %, respectively). Regarding the type of publication, PEG tube placement-related bleeding occurred more frequently in research published as abstracts than those in full papers (4.91 % vs. 1.95 %, respectively). With regard to aspirin doses, only two studies (18,30) reported on low (< 350 mg daily) and high (> 350 mg daily) aspirin doses, with the remaining documents providing no information on this aspect, which prevented this study from undertaking differential analysis.
Discussion
This meta-analysis of 11 published documents demonstrates that maintaining antiplatelet therapy is safe in patients undergoing PEG-tube insertion, since the risk of procedural-related bleeding was not increased in comparison to patients receiving no medication. Additionally, the overall risk of bleeding after PEG insertion was low, with less than 4 % of patients presenting this complication. When bleeding did occur, the severity of the episode was mild or moderate, and resolved after conservative or endoscopic treatment, with no need of surgical intervention. There was no attributable death in any of the cases reported in the documents retrieved.
Different international guidelines recommend discontinuation of antiplatelet therapy at least 7-10 days prior to any high risk endoscopic procedure. Interestingly, although none of the studies researched followed the current guidelines for the management of antithrombotic therapy because they would have been outside the "Inclusion Criteria", no significant differences in the frequency or type of PEG-placement-related complication between groups were noted, and no major bleeding complication was documented.
The decision to withhold or continue antithrombotic therapy is based on the type of procedure and on each patient's risk of developing thromboembolic cardio or cerebrovascular events. Aspirin and thienopyridines decrease platelet aggregation by either irreversibly inhibiting platelets' cyclooxigenase or blocking the adenosine biphosphate receptor, respectively, disabling the platelets through their life span of about 7-10 days (30). This study's inclusion criteria considered patients who received these drugs up to 2 days before PEG placement, so we can consider that they were under the full effects of such medication at the time of the endoscopic procedure. The results of this research are consistent with the fact that PEG placement can be considered a safe procedure in patients who are under treatment with aspirin, clopidogrel and dual antiplatelet treatment, with no increased risk of procedural-related bleeding in comparison to patients who received no such treatment.
This study's results are particularly relevant at a time when demographic trends show an increasing incidence of cardiovascular disease and cerebral ischemia, together with a progressively aging population in developed countries. Additionally, the main indications for PEG in both Europe and US are cerebrovascular diseases, stroke and neurodegenerative and other neurological diseases (37-40). Most of the patients undergoing PEG also have multiple comorbidities, and individual embolism and thrombotic risks may play a major role when deciding whether to stop anti-thrombotic therapy in order to avoid deleterious events. These concerns could provide an explanation as to why ASGE and BGS recommendations are not universally followed in many cases. Fortunately, an accumulated body of knowledge has provided data for research on the true risk of potential bleeding when not following the standard recommendations.
One relevant finding of our meta-analysis was the consistency between the results of each antiplatelet treatment option assessed. Results from aspirin and clopidogrel showed a great deal of homogeneity (with I2 equaling 0 %), which indicates that it is possible to generalize the results. For dual antiplatelet therapy-based studies the homogeneity was near moderate (I2 equaling 47 %), reflecting the limited number and size of studies that were assessed for this.
The strength of this research lies in the fact that it compiles the results of an exhaustive literature search in 3 major databases, and in abstracts books of the 3 major Gastroenterology congresses. Furthermore, recovered studies were critically appraised according to their methodological aspects, and different investigators independently extracted the data from the studies included. Nevertheless, the possibility of not recovering all the relevant information published on antiplatelet-attributable risk of bleeding in patients undergoing PEG tube placement should be considered as one of the limitations of the study, along with a risk of bias that may remain in spite of having excluded any such publication bias by means of a funnel plot analysis and appropriate statistical tests. In addition, most of the studies included in this systematic review were retrospective and observational, as no randomized clinical trials were retrieved. Although this meta-analyses of predominantly retrospective studies show that aspirin, clopidogrel and dual antiplatelet therapy do not increase the risk of PEG tube insertion-induced bleeding, the power of these studies is limited and the results treated with caution therefore. The search strategy also retrieved documents reporting on bleeding risk associated with PEG tube placement but since this was not a primary outcome of these studies, the results were not optimized. However, adverse events related to such an invasive procedure as PEG tube insertion, including a bleeding episode, were relevant enough to have been properly registered on patients' charts thus this information was not lost.
This analysis has tried to solve some of the limitations presented by the moderate quality of much of the data retrieved through the use of the more conservative statistical analysis random effects models, (despite the high homogeneity of the results which would have allowed for the application of fixed-effect models tests). Even with this strategy, the results show that the risk of bleeding in patients undergoing PEG tube insertion did not significantly increased with regard to controls for any antiaggregant treatment use or combination.
In conclusion, our research has shown that PEG tube insertion is a safe procedure for patients who are undergoing antiplatelet treatment, with no increased procedural-related bleeding risk when compared to control patients. Such a result allows for the avoidance of exposing patients to potential thromboembolic risks as a result of withdrawal from this medication when used for primary or secondary prevention of cardio and cerebrovascular diseases. Further well designed prospective research is needed to confirm the results from this predominantly retrospective data based meta-analysis.
Acknowledgements
We would like to thank Dr. Mihai Paduraru and Melanie Louise Radcliff for their help in linguistic content review and insight in this study methodology.
References
1. Lucendo AJ, Friginal-Ruiz AB. Percutaneous endoscopic gastrostomy: an update on its indications, management, complications, and care. Rev Esp Enferm Dig 2014;106:458-65. [ Links ]
2. Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: A percutaneous endoscopic technique. J Pediatr Surg 1980;15:872-5. [ Links ]
3. Shaver WA, Winer SF, Nyder EJ. Gastrostomy: A simple and effective technique. South Med J 2014;81:719-23. [ Links ]
4. Laskaratos FM, Walker M, Gowribalan J, Gkotsi D, Wojciechowska V, Arora A, et al. Predictive factors for early mortality after percutaneous endoscopic and radiologically-inserted gastrostomy. Dig Dis Sci 2013;58:3558-65. [ Links ]
5. Zuercher BF, Grosjean P, Monnier P. Percutaneous endoscopic gastrostomy in head and neck cancer patients: Indications, techniques, complications and results. Eur Arch Otorhinolaryngol 2011;268:623-9. [ Links ]
6. Miller RE, Kummer BA, Tiszenkel HI, Kotler DP. Percutaneous endoscopic gastrostomy: Procedure of choice. Surg Endosc 1986;204:543-5. [ Links ]
7. Ditchburn L. The principles of PEG feeding in the community. Nurs Times 2006;102:43-5. [ Links ]
8. Pruthi D, Duerksen DR, Singh H. The practice of gastrostomy tube placement across a Canadian regional health authority. Am J Gastroenterol 2010;105:1541-50. [ Links ]
9. Tokunaga T, Kubo T, Ryan S, et al. Long-term outcome after placement of a percutaneous endocopic gastrostomy tube. Geriatr Gerontol Int 2008;8:19-23. [ Links ]
10. Luman W, Kwek KR, Loi KL, Chiam MA, Cheung MA, Ng HS. Percutaneous endoscopic gastrostomy - indications and outcome of our experience at the Singapore General Hospital. Singapore Med J 2001;42:460-5. [ Links ]
11. Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am 1996;6:409-22. [ Links ]
12. Veitch AM, Baglin TP, Gershlick AH, Harnden SM, Tighe R, Cairns S, et al. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic procedures. Gut 2008;57:1322-9. [ Links ]
13. ASGE Standards of Practice Committee, Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc 2009;70:1061-70. [ Links ]
14. Dong P, Yang XC, Bian SY. Should antiplatelet therapy be interrupted in drug eluting stent recipients throughout the periendoscopic period? A very late stent thrombosis case report and review of the literature. J Geriatr Cardiol 2014;11:274-7. [ Links ]
15. Taylor-Sutton JE, Kim MC. Very late stent thrombosis approximately 7 years after deployment and one-week cessation of dual antiplatelet therapy. J Invasive Cardiol 2011;23:E273-6. [ Links ]
16. Macellari F, Paciaroni M, Agnelli G, Caso V. Perioperative stroke risk in nonvascular surgery. Cerebrovasc Dis 2014;34:175-81 [ Links ]
17. Diehl P, Halscheid C, Olivier C, Helbing T, Bode C, Moser M. Discontinuation of long term clopidogrel therapy induces platelet rebound hyperaggregability between 2 and 6 weeks post cessation. Clin Res Cardiol 2011;100:765-71. [ Links ]
18. Richter JA, Patrie JT, Richter RP, Henry ZH, Pop GH, Regan KA, et al. Bleeding after percutaneous endoscopic gastrostomy is linked to serotonin reuptake inhibitors, not aspirin or clopidogrel. Gastrointest Endosc 2011;74:22-34. [ Links ]
19. Singh D, Laya AS, Vaidya OU, Ahmed SA, Bonham AJ, Clarkston WK. Risk of bleeding after percutaneous endoscopic gastrostomy (PEG). Dig Dis Sci 2012;57:973-80. [ Links ]
20. Ruthmann O, Seitz A, Richter S, Marjanovic G, Olschewski M, Hopt UT, et al. Percutaneous endoscopic gastrostomy. Complications with and without anticoagulation. Chirurg 2010;81:247-54. [ Links ]
21. Lee C, Im JP, Kim JW, Kim SE, Ryu DY, Cha JM, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: A multicenter, retrospective study. Surg Endosc 2013;27:3806-15. [ Links ]
22. Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: A case series of 1041 patients. Can J Gastroenterol 2011;25:201-6. [ Links ]
23. Stroup DF, Berlin JA, Morton SC, Olkin I, Willamson GD, Rennied D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [ Links ]
24. Urrútia G, Bonfill X. PRISMA declaration: a proposal toimprove the publication of systematic reviews and metaanalyses. Med Clin (Barc) 2010;135:507-11. [ Links ]
25. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vanderbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiol 2007;18:800-4. [ Links ]
26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [ Links ]
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [ Links ]
28. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443-57. [ Links ]
29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [ Links ]
30. Lozoya-González D, Pelaez-Luna M, Farca-Belsaguy A, Salceda-Otero JC, Vazquéz-Ballesteros E. Percutaneous endoscopic gastrostomy complication rates and compliance with the American Society for Gastrointestinal Endoscopy guidelines for the management of antithrombotic therapy. JPEN J Parenter Enteral Nutr 2012;36:226-30. [ Links ]
31. Bal BS, Karin AA, Olden KW. Percutaneous endoscopic gastrostomy placement: Do we need to stop the antiplatelet therapy? Gastrointest Endosc 2011;73(Supl. 4):AB226. [ Links ]
32. Sharma K, Krishnamurthy P. laceration of gastroepiploic artery from percutaneous endoscopic gastrostomy with severe intra-peritoneal hemorrhage. Am J Gastroenterol 2012;107(Supl. 1):S269-70. [ Links ]
33. Thosani N, Nevah MI, Khanijow V, Machicado JD, Fallon MB, Dupont AW, et al. Bleeding risk associated with continuing antiplatelet therapy during PEG tube placement: should we throw caution to the wind? Gastrointest Endosc 2012;75(Supl. 4):AB243. [ Links ]
34. Meier A, Gölder SK, Probst A, MessmannIII H. The risk of bleeding performing percutaneous endoscopic gastrostomy (PEG) undergoing antiplatet therapy - a single center experience. Gastrointest Endosc 2012;75(Supl. 4):AB370. [ Links ]
35. Thosani N, Nevah M, Batra S, Khanijow V, Machicado JD, Spinn M, et al. Bleeding risk associated with uninterrupted clopidogrel therapy during PEG tube placement: largest single center experience. Am J Gastroenterol 2012;107(Supl. 1):S777. [ Links ]
36. Im JP, Cha JM, Kim JW, Kim SE, Ryu DY, Kim EY, et al. Proton pump inhibitor use before percutaneous endoscopic gastrostomy is associated with adverse outcomes. Gut Liver 2014;8:248-53. [ Links ]
37. Hebuterne X, Bozzetti F, Moreno Villares JM, Pertkiewicz M, Shaffer J, Staun M, et al. Home enteral nutrition in adults: a European multicentre survey. Clin Nutr 2003;22:261-6. [ Links ]
38. Callahan CM, Haag KM, Weinberger M, Tierney WM, Buchanan NN, Stump TE, et al. Outcomes of percutaneous endoscopic gastrostomy among older adults in a community setting. J Am Geriatr Soc 2000;48:1048-54. [ Links ]
39. Ermis F, Ozel M, Oncu K, Yazgan Y, Demirturk L, Gurbuz AK, et al. Indications, complications and long-term follow-up of patients undergoing percutaneous endoscopic gastrostomy: A retrospective study. Wien Klin Wochenschr 2012; 124: 148-53. [ Links ]
40. Kurien M, Westaby D, Romaya C, Sanders DS. National survey evaluating service provision for percutaneous endoscopic gastrostomy within the UK. Scand J Gastroenterol 2011;46:1519-24. [ Links ]
![]() Correspondence:
Correspondence:
Alfredo J Lucendo
Department of Gastroenterology
Hospital General de Tomelloso
Vereda de Socuéllamos, s/n
13700 Tomelloso, Ciudad Real. Spain
e-mail: alucendo@vodafone.es
Received: 17-12-2014
Accepted: 18-01-2015