My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.6 Madrid Jun. 2015
ORIGINAL PAPERS
Drug consumption and additional risk factors associated with microscopic colitis: Case-control study
Danila Guagnozzi1, Alfredo J. Lucendo1, Teresa Angueira1, Sonia González-Castillo1 and José María Tenias2
1Department of Gastroenterology. Hospital General de Tomelloso. Tomelloso, Ciudad Real. Spain.
2Research Support Unit. Hospital General La Mancha Centro. Alcázar de San Juan, Ciudad Real. Spain
ABSTRACT
Background: Microscopic colitis has now emerged as a common cause of chronic diarrhoea, but its aetiology remains unknown. Some studies suggest that commonly prescribed drugs and other additional risk factors may be triggers.
Aims: To evaluate the effects of drug intake and other risk factors on microscopic colitis patients.
Methods: A prospective, case-control study with all consecutive adult patients referred to the Hospital General de Tomelloso (Ciudad Real, Spain) for chronic watery diarrhoea (from 2008 to 2011) was performed. Microscopic colitis was diagnosed following the commonly accepted histopathological criteria.
Results: 46 consecutive new cases of microscopic colitis and 317 chronic diarrhoea controls were recruited. Five independent risk factors significantly associated with microscopic colitis were identified: Abdominal pain (OR 3.25; 95%CI, 1.49-7.08), weight loss (OR 2.67; 95%CI, 1.16-6.15), celiac disease (OR 15.3; 95%CI, 3.70-63.5), topiramate intake (OR 13.6; 95%CI, 1.84-100.8), and older age at diagnosis (OR 1 year increase 1.022; 95%CI, 1.002-1.042). Use of non-steroidal anti-inflammatory drugs was associated with microscopic colitis in the subgroup of patients who fulfilled irritable bowel syndrome criteria (38.5% vs. 10.8%; p < 0.017).
Conclusions: Microscopic colitis is associated with autoimmune disease, an increased age at diagnosis, topiramate intake and only in a sub-group of irritable bowel disease patients with non-steroidal anti-inflammatory drugs.
Key words: Microscopic colitis. Drug intake. Irritable bowel syndrome. Chronic diarrhoea.
Introduction
Microscopic colitis (MC) is a term used to identify a group of chronic inflammatory bowel disorders characterised by chronic or recurrent watery diarrhoea in the absence of abnormal radiological examinations, with normal or near-normal endoscopic appearance, and specific microscopic abnormalities in colonic biopsies (1-3). The disorder comprises two mayor subtypes: lymphocytic colitis (LC) and collagenous colitis (CC), which are clinically indistinguishable, but which present different histopathological features (4,5). Although several variants of these two conditions have been reported, they are probably not specific entities (6).
Both the incidence and prevalence of MC have increased over time. Once considered a rare disease (7), MC is now common, with a high incidence and a very high prevalence (8-16). Nevertheless, its etiology remains unknown. Commonly held theories propose that environmental factors such as infection, toxins, or drugs may induce an abnormal activation of the immune response to luminal antigens, which in turn leads to inflammation of the colonic mucosa (7). Drug consumption has been considered an environmental risk factor implicated as a causative or triggering agent of MC. Indeed, significant associations werereported between MC and certain drugs, including non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), selective serotonin re-uptake inhibitors (SSRIs), beta blockers, statins, and bisphosphonates, although various studies gave conflicting results (17-26). The possible causative nature of these associations is a matter of ongoing discussion and research. The drugs and their metabolites can affect the colon directly, either through their pharmacological activity or through idiosyncratic hypersensitivity reactions they cause in the colonic mucosa, or indirectly by altering the colonization of gastrointestinal organisms (6). Several drugs thought to be associated with MC are also known to be associated with the development of chronic or recurrent diarrhoea as a side effect (27), further confusing the possible causal role of drugs in the development of the disease. Whereas retrospective data show a significant association in some studies, prospective data is still lacking.
In order to shed light on this issue, this study aims to evaluate the prevalence of drug intake and other risk factors in patients with LC and CC compared to a control group of patients with chronic or recurrent diarrhoea.
Patients and methods
Study setting
We performed a prospective, single-centre, case-control study with all consecutive adult patients referred to the Department of Gastroenterology at the Hospital General de Tomelloso (located in central Spain in the autonomous region of Castilla-La Mancha) for chronic or recurrent watery diarrhoea (defined by persistent symptoms lasting over 1 month) between April 2008 and December 2011. A complete colonoscopy carried out under conscious sedation was performed on all patients, reaching the cecum in all cases. At least two biopsy samples were obtained with the aid of a standard, open-type endoscopic biopsy forceps in each of the following colonic sections: a) Cecum and/or ascending colon; b) transverse colon; and c) descending and sigmoid colon.Biopsies were fixed separately in 4% formalin and routinely processed. The colonic biopsy specimens were stained with haematoxylin-eosin.
All patients with stool samples testing positive for ova, parasites, Clostridium difficile toxin, Salmonella spp, Shigella spp, Campylobacter, or Yersinia were excluded, as were those with a previous diagnosis of celiac disease (CD), inflammatory bowel disease (IBD), or any other known intestinal disease. Patients with a positive serology for human immunodeficiency virus were also excluded from the study, as were patients who had undergone previous gastrointestinal or colonic surgery (except appendectomy or cholecystectomy). In addition, we excluded women who were pregnant or breast-feeding at the moment of clinical evaluation.
Case and control group definitions
We identified consecutive cases of adult patients with a new diagnosis of LC or CC as confirmed through colonic biopsies. Differential diagnoses of CC versus LC were made by an expert pathologist based on histopathological criteria. CC was defined by: A diffusely distributed and thickened sub-epithelial collagen layer (> 10 µm), epithelial damage such as flattening and detachment, inflammation in the lamina propria with mainly mononuclear cells, and an increased number of intraepithelial lymphocytes (IEL). In particular, cases of CC were identified by measuring the sub-epithelial collagen layer after van Gieson staining with the aid of an ocular micrometer in a representative and well-orientated section of the mucosa, where three adjacent crypts were cut vertically, extending all the way down to the muscularis mucosa. In doubtful cases, Masson Trichrome staining was carried out to optimally assess the existence of a collagen layer. LC was defined by: IEL > 20 per 100 surface epithelial cells (28), epithelial damage such as flattening and mucin depletion, inflammation in the lamina propria with mainly mononuclear cells, and a sub-epithelial collagen layer < 10 µm. LC cases were identified by counting IEL. The number of IEL per 100 epithelial cells was calculated by counting the number per 300 epithelial cells divided by three. Only the surface epithelium was examined, avoiding areas overlying lymph follicles in the lamina propria. Counting was performed on at least two biopsy samples from different sections of the colon and the mean number for each patient was recorded. Counting of IEL was performed in sections stained with haematoxylin-eosin; in those cases in which the IEL count resulted not conclusive, CD3 immunohistochemistry staining was used. The severity of epithelial cell damage as well as inflammation of the lamina propria were estimated. Occasional crypt abscesses, cryptitis, crypt distortions, or neutrophil leucocytes in the lamina propria were allowed (19,20).
We selected a concurrent control group composed of all consecutive adult patients with watery chronic or recurrent diarrhoea who presented both a normal colonoscopy and normal histopathology in colon biopsies (≤ 5 IEL/100). We studied the prevalence of CD in all patients by determining serological CD-related antibodies (immunoglobulin A [IgA] tissue transglutaminase antibodies and total IgA) and by assessing histopathological features in duodenal biopsies (on at least six biopsy samples from the duodenal bulb and second/third duodenum segments) according to both the Marsh classification (28) and the new simplified Corazza classification (30). Where necessary, genetic markers (haplotype HLA) and rechallenge protocol after a gluten free diet were also carried out.
Clinical data
A detailed medical history was perform in each patient, including clinical data, family history of chronic intestinal disease (CD, IBD, colorectal cancer, or cancer in any other part of the intestinal tract), lifestyle factors (smoking was divided into three categories: Current smoker, former smoker, and non-smoker), concomitant chronic diseases, and complete chronic or frequent drug intake (defined as more than 3 days/week) up to 3 months before carrying out the colonoscopy (18). All drugs were recorded separately and analysed. The type of symptoms and the appearance of anaemia and/or increased inflammatory markers were also noted. Every MC and control patient was classified into two groups according with they met or not the Rome III criteria (31) for a diagnosis of diarrhoea-dominant irritable bowel syndrome (IBS) or functional chronic diarrhoea without the appearance of "alarm symptoms", including age ≥ 50 years old. The "alarm symptoms" considered were as follows: unexplained weight loss (>10 lbs over 6 months), fever, significant gastrointestinal bleeding (a spotting of red blood on the toilet tissue after a bowel movement was not considered to be an alarm symptom), increased inflammatory markers, presence of iron deficiency anaemia, and/or those patients who reported a family history of a first degree relative with colon cancer, CD, or IBD. In patients with a diagnosis of CC or LC, the date of diagnosis was defined as the date on which the colonoscopy was performed.
Data analysis and ethics
Results were expressed as mean plus standard deviation (quantitative variables) or as percentages (qualitative variables). The differences in clinical characteristics depending on age group were explored with the aid of the chi-square test (categorical variables) and an analysis of variance (quantitative variables). When appropriate, nonparametric tests were used, namely Fisher's exact test and the Mann-Whitney test. Values of p < 0.05 were considered to be statistically significant. Associations with MC were estimated as Odds Ratios (OR) and 95% confidence intervals (95%CI). Associations were adjusted by means of logistic regression.
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the institutional review board of our hospital. Informed consent was obtained from all patients.
Results
Patient characteristics
Data on the characteristics of both the MC patients and the control group are given in table I. During the study period, 46 consecutive new cases of MC (25 females and 21 males) were included (4 with CC and 42 with LC). For evaluating the characteristics of patients with MC, we considered them to be a single group since the number of patients with CC (4) was too small to be compared with that of LC patients (42). The mean age at diagnosis of MC was 50.2 ± 20.2 years, with 10.9% (5/46) of the subjects being over 70 years old and 17.4% (8/46) under the age of 30. Mucosal appearance in colonoscopies was normal in all patients with a diagnosis of MC. With regard to the extent of colonic involvement, 28% of MC patients only had one section affected in the right colon while the remaining subjects presented more diffuse involvement affecting all colonic segments. Associated symptoms were observed in 52.2% (24/46) of MC patients, with a higher prevalence of abdominal pain and weight loss. Increasing values of inflammatory markers were present in only 8.7% (4/46) of MC patients; even fewer 6.5% (3/46) showed signs of iron deficiency anaemia. CD was observed in 13% (6/46) of patients with MC.
The control group consisted of 317 patients (196 female and 121 male; median age: 48.6 ± 17.8 years) consecutively diagnosed with chronic or recurrent diarrhoea. There were no significant differences in median age, sex, cases fulfilling the Rome III criteria for diarrhoea-dominant IBS, or associated symptoms between the case and control groups except for the presence of abdominal pain and weight loss, which was significantly associated with a diagnosis of MC compared to controls (34.8% vs. 14.5%, p < 0.05 and 26.1% vs. 10.4%, p < 0.05, respectively). We observed only 3.8% (12/317) patients in the control group fulfilling the Rome III criteria for functional chronic diarrhoea without observing any patients in the MC group. No statistically significant differences were observed between the MC and control groups with respect to any other associated symptoms.
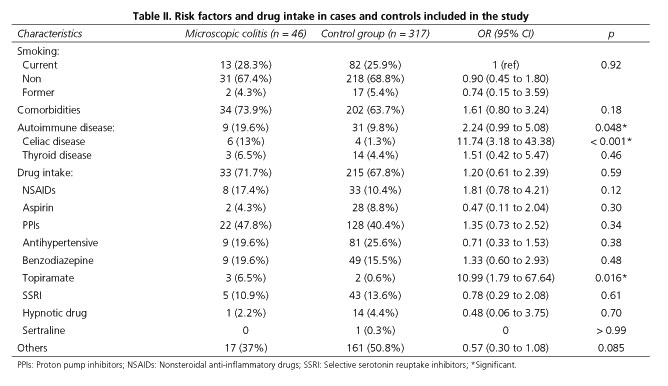
The data on different risk factors for MC evaluated in the study are shown in table II. We found that the presence of autoimmune diseases (19.6% vs. 9.8%, p < 0.05), particularly CD, was significantly more frequent in patients diagnosed with MC as compared to the control group (13% vs. 1.3%, p<0.05) (Table II). The presence of CD was observed in 13% (6/46) of patients with MC and all patients presented a LC type associated. All celiac patients showed the presence of HLA-DQ2 and/or HLA-DQ8 and the absence of negative IgA tissue transglutaminase antibody serum levels without IgA deficiency. According to the Marsh classification and the new simplified classification, we identified five patients with Marsh I-II/Stage A and one patient with Marsh 3a-3b/Stage B1. All patients presented a normalization of duodenal mucosal biopsies after six month with gluten-free diet and subsequent reappearance of the lesions in the duodenal mucosa after gluten-diet reintroduction during 6 months. In the control group, 1.3% of patients had CD (4/317), with all patients presenting HLA-DQ2 and/or DQ8 and negative IgA tissue transglutaminase antibody serum levels without IgA deficiency. We found three patients with Stage A and one patient with Stage B1.
The presence of comorbidities was not significantly associated with MC when compared to the control group; smoking was likewise not a significant factor (considering smokers at the time of diagnosis, non-smokers, and former smokers) (Table II).
Drug consumption
The majority of patients had been prescribed at least one medication during the year prior to the colonoscopy (71.7% in the MC group and 67.8% in the control group, p = not significant), with the same average number of daily drugs consumed in the MC and control groups. Analysing all medications separately, we found no difference in drug intake between the MC and control groups except in the case of topiramate, which was consumed more frequently in MC patients (6.5% in the MC vs. 0.6% in the control group, p = 0.016) (Table II). It is important to emphasize that all patients with MC who were taking topiramate were under 30 years of age (median age: 21 years; range: 24-19) with a main indication for topiramate intake of bipolar disorder, migraines or epilepsy. The percentage of NSAIDs prescribed during the same time period was similar between the two groups (17.4% vs. 10.4%, respectively), but when the sub-group of patients who fulfilled the Rome III criteria was analysed separately, we observed a significant association between the intake of NSAIDs and MC patients as compared to the control group (38.5% vs. 10.8%, p < 0.017). The same association was documented among the subgroup of patients aged < 50 years old (20.8% in MC vs. 8.9% in controls) compared to those ≥ 50 years old (13.6% in MC vs. 12.2% in controls). The majority of patients in the first group (85%) fulfilled Rome-III criteria for diarrhoea-dominant IBS. The main indications in young patients for NSAIDs intake were migraine and chronic arthropathy without inflammatory characteristics. Considering the sub-group of patients under 50, a significant association between NSAIDs and MC was confirmed. There was also a higher prevalence of metamizol intake in the MC group, but the difference was not statistically significant (50% vs. 23.3%, p = 0.12) (Table III). PPIs and SSRIs were used by 40.4% and 13.6% of MC patients, and 47.8% and 10.9% of the controls, respectively, with no statistical differences between the two groups. In particular, considering the different types of PPIs prescribed, only a higher intake of lansoprazole was observed in MC patients compared to controls, but this difference did not reach statistical significance (9.1% vs. 5.5%, p = 0.72).
The final logistic regression model revealed five independent risk factors for MC: Associated presenting symptoms (such as abdominal pain and weight loss), CD, topiramate intake, and increased age at diagnosis (Table IV). It is important to highlight that the association between age and MC was more marked in patients over 75 as compared with younger patients (adjusted OR: 4.50; 95% CI, 1.76-11.5).
Discussion
While the exact aetiology of MC remains unknown, leading models of its pathogenesis point to an immune or inflammatory response to luminal factors and medications (2,7), although no dominant mechanism has emerged.
In our case-control study we confirmed the previously described association of MC with autoimmune disease, especially with CD, though the association with MC is still controversial because the colonic lymphocytosis could represent an epiphenomenon of CD or a real association of separate entities. In our study positive anti-tissue transglutaminase antibodies were not detected in patients with MC and conconmitant CD, suggesting that sprue serology could be less sensitive in MC patients as previously reported (32). For this reason, CD diagnoses were confirmed by a rechallenge protocol after inducing disease remission with a gluten-free diet. Moreover we found a new significant association between MC and the intake of topiramate, an anticonvulsant drug used to treat epilepsy, bipolar disorder, migraines, and recently approved by the Food and Drug Administration for weight loss. It is important to emphasize that, to the best of our knowledge, this is the first report of a possible association between topiramate intake and MC in a case-control study, despite we were not able to establish a casual relationship. Furthermore, considering the small sample size of our study we should consider this novel association as preliminary data that need a further confirmation in a larger sample size study. The association of MC with another antiepileptic drug as carbamazepine has been previously described in two case reports (33,34). Despite the fact that these drugs having distinctive mechanisms of action, both share a common one by blocking a sodium channel, which could probably being implicated in producing diarrhoea and MC appearance. Interestingly, in our study all patients taking topiramate with MC were very young, with a median age of 21 years. Although MC can occur in patients of any age, previously published studies seemed to indicate that it was more common among older patients (10-12,35,36). However, other studies have shown that 25% of all MC patients are under the age 45 and even some paediatric cases have been reported (36-38). In our study, the median age at diagnosis of MC was lower in comparison to other studies (50.2 ± 20.2 years old), with 17.4% of patients diagnosed under the age of 30. Nevertheless, our results show that increased age at the time of diagnosis remains an independent risk factor for the development of MC. This finding suggests that the etiopathogenetic mechanisms involved may be different in a specific sub-group of MC patients, with a different age of disease development and different risk factors. Greater attention should be given to younger patients with MC, for whom it is a newly emerging disease requiring increased clinical awareness. Moreover, when assessing a sub-group of patients under the age of 50 who fulfilled the Rome III criteria, we observed a significant association between MC and NSAID intake, as reported previously. In contrast, we observed no increased risk of MC in association with other drugs as reported in several previous studies. We separately analysed MC patients fulfilling diagnostic criteria of IBS, because a higher use of certain medication, including antidepressants, acid inhibitors agents and other several drugs associated with MC, has been also demonstrated among patients with IBS compared with controls from the general population (39). Therefore the separated analysis can help to distinguish the role of drug intake in the diarrhoea of functional characteristics and diarrhoea of MC.
It is important to note that data on the association between drug intake and MC remain controversial. In one systematic review, 17 drugs, including some of the most frequently prescribed medications today (PPIs, SSRIs, and statins), were determined to be "high" in terms of likelihood of causality (40); however, most of this data came from uncontrolled observations. The majority of studies on this topic have been in the form of case series, often reporting improvement after drug withdrawal and recurrence with re-challenge, thus supporting the supposed etiological role of the medication. Previous case-control studies have also shown the association between MC and drug intake, mainly for aspirin, NSAIDs, lansoprazole, omeprazole, and sertraline use (8,17-20). However, the only report based on data from a prescription database found no association between development of MC and drug consumption (21). In another study comparing MC cases to a chronic diarrhoea group, the use of several drugs presumably associated with MC showed no significant differences between groups (18).
In this study we observed no association between MC diagnosis and smoking. The role of smoking in the development of MC has already been studied in three cohort studies. The first one revealed that smoking was significantly more frequent in patients with CC than in controls, with smokers developing the disease 10 years earlier than non-smokers (41,42). These results were corroborated in a recent Spanish study (20). In another study, more MC patients than controls were smokers, with a former or current smoker yielding an OR of 2.12 (1.56-2.88). This risk was more prominent in current smokers (OR of 5.36, 3.81, and 4.37 for CC, LC, and all MC, respectively; 95% CI all greater than 1) (43). In these studies the association with smoking was higher for CC than LC. It is therefore likely that the lack of an association observed in our study was due to the high prevalence of LC in the case group (91.3%). Furthermore, the small sample size of cases may have affected the statistical analysis.
Our study has several limitations. As mentioned above, the study was carried out with a small sample size of cases that may limit the study's statistical power for identifying possible associations and differences between the different subtypes of MC. In our study we considered the patients with LC and CC to be a single group since the number of patients with CC was too small to be compared with that of LC patients in our sample population (16). Considering the selection of control group, the healthy volunteers would have been the optimal controls, but ethical and practical considerations prevented us to select them such as controls. Accordingly we select as a control group the patients with chronic watery diarrhoea of functional characteristics (normal colonoscopy and colonic biopsies) as has already been used in a previous published case-control studies (1). In addition, we were not able to evaluate the association of MC on the basis of each specific class of medication prescribed because of the small number of case subjects. Future studies should use a larger sample size to better define the association between drug intake and specific sub-groups of MC patients.
In summary, we found a significant association between MC and autoimmune disease along with a new, significant association with topiramate intake, especially in younger patients with MC. Only in one sub-group of patients under the age of 50 and fulfilling the diagnostic criteria for IBS did we observe a significant association between the intake of NSAIDs and MC as compared to the control group, an association which had been reported previously. This result may reflect different rates of exposure to MC-inducing agents in different sub-groups of patients with MC. In addition, our results reinforce the idea that MC can be considered an umbrella term for different entities that should perhaps be studied separately. Also, with regard to age groups, our results indicate that special consideration should be given to younger patients. To gain further insight into this subject, more prospective studies are needed to identify the different etiopathogenetic mechanisms that correlate to various phenotypic characteristics.
References
1. Jawhari A, Talbot JC. Microscopic, lymphocytic and collagenous colitis. Histopathology 1996;29:101-10. [ Links ]
2. Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology 2011; 140:1155-65. [ Links ]
3. Guagnozzi D, Lucendo AJ. Advances in knowledge on microscopic colitis: From bench to bedside. Rev Esp Enferm Dig 2015;107:98-108. [ Links ]
4. Zins BJ, Sandborn WJ, Tremaine WJ. Collagenous and lymphocytic colitis: Subject review and therapeutic alternatives. Am J Gastroenterol 1995;90:1394-400. [ Links ]
5. Fernandez-Bañares F, Salas A, Esteve M, et al. Collagenous and lymphocytic colitis: Evaluation of clinical and histological features, response to treatment and long-term follow-up. Am J Gastroenterol 2003;98:340-7. [ Links ]
6. Geboes K, Villanacci V. Terminology for the diagnosis of colitis. J Clin Pathol 2005;58:1133-4. [ Links ]
7. Münch A, Aust D, Boohr J, et al; European Microscopic Colitis Group (EMCG). Microscopic colitis: Current status, present and future challenges. J Crohn's Colitis 2012;6:932-45. [ Links ]
8. Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence and temporal trends of microscopic colitis: A systematic review and meta-analysis. Am J Gastroenterol 2015;110:165-76. [ Links ]
9. Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: A common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut 2004;53:346-50. [ Links ]
10. Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis in Orebro, Sweden, an epidemiological study 1984-1993. Gut 1995;37:394-7. [ Links ]
11. Fernandez-Bañares F, Salas A, Forné M, et al. Incidence of collagenous and lymphocytic colitis: A 5-years population-based study. Am J Gastroenterol 1999;94:418-23. [ Links ]
12. Agnarsdottir M, Gunnlaugsson O, Orvar KB, et al. Collagenous and lymphocytic colitis in Iceland. Dig Dis Sci 2002;47:1122-8. [ Links ]
13. Pardi DS, Loftus EV Jr, Smyrk TC, et al. The epidemiology of microscopic colitis: A population based study in Olmsted Country, Minnesota. Gut 2007;56:504-8. [ Links ]
14. Williams JJ, Kaplan GG, Makhija S, et al. Microscopic colitis defining incidence rates and risk factors: A population-based study. Clin Gastroenterol Hepatol 2008;6:35-40. [ Links ]
15. Fernandez-Bañares F, Salas A, Esteve M, et al. Evolution of the incidence of collagenous colitis and lymphocytic colitis in Terrassa, Spain: A population-based study. Inflamm Bowel Dis 2011;17:1015-20. [ Links ]
16. Guagnozzi D, Lucendo AJ, Angueira-Lapeña T. Prevalence and incidence of microscopic colitis in patients with diarrhoea of unknown aetiology in a region in central Spain. Dig Liver Dis 2012;44:384-8. [ Links ]
17. Keszthelyi D, Jansen SV, Schouten GA, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: A case-control study. Aliment Pharmacol Ther 2010;32:1124-8. [ Links ]
18. Fernandez-Bañares F, Esteve M, Espinós JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol 2007;102:324-30. [ Links ]
19. Fernandez-Bañares F, Sousa MR, Salas A, et al; RECOMINA Project, GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa). Impact of current smoking on the clinical course of microscopic colitis. Inflamm Bowel Dis 2013;19:1470-6. [ Links ]
20. Riddell RH, Tanaka M, Mazzoleni G. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: A case-control study. Gut 1992;33:683-6. [ Links ]
21. Pascua MF, Kedia P, Weiner MG, et al. Microscopic colitis and medication use. Clin Med Insights Gastroenterol 2010;3:11-9. [ Links ]
22. Beaugerie L, Patey N, Brousse N. Ranitidine, diarrhoea, and lymphocytic colitis. Gut 1995;37:708-11. [ Links ]
23. Piche T, Raimondi V, Schneider S, et al. Acarbose and lymphocytic colitis. Lancet 2000;356:1246. [ Links ]
24. Beaugerie L, Luboinski J, Brousse N, et al. Drug induced lymphocytic colitis. Gut 1994;35:426-8. [ Links ]
25. Wilcox GM, Mattia A. Collagenous colitis associated with lansoprazole. J Clin Gastroenterol 2002;34:164-6. [ Links ]
26. Wilcox GM, Mattia AR. Microscopic colitis associated with omeprazole and esomeprazole exposure. J Clin Gastroenterol 2009;43:551-3. [ Links ]
27. Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf 2000;22:53-72. [ Links ]
28. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827-51. [ Links ]
29. Marsh MN. Gluten major histocompatibility complex, and the small intestine. A molecular and immunological approach to the spectrum of gluten sensity ("celiac sprue"). Gastroenterology 1992;102:330-54. [ Links ]
30. Corazza GR, Villanacci V. Coeliac disease. J Clin Pathol 2005;58:573-4. [ Links ]
31. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480-9. [ Links ]
32. Bohr J, Tysk C, Yang P, et al. Autoantibodies and immunoglobulins in collagenous colitis. Gut 1996;39:73-6. [ Links ]
33. Maroy B. Acute lymphocytic colitis due to carbamazepine. Gastroenterol Clin Biol 2010;34:155-6. [ Links ]
34. Alvarez-Pérez P, Rubio-Nazabal E, Marey-López J, et al. Colitis linfocitaria inducida por carbamacepina.An Med Interna 2004;21:572-3. [ Links ]
35. Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: A retrospective clinical study of 199 Swedish patients. Gut 2004;53:536-41. [ Links ]
36. Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: A retrospective study of clinical presentation and treatment in 163 patients. Gut 1996;39:846-51. [ Links ]
37. Gremse DA, Boudreaux CW, Manci EA. Collagenous colitis in children. Gastroenterology 1993;104:906-9. [ Links ]
38. Mahajan L, Wyllie R, Goldblum J. Lymphocytic colitis in a paediatric patients: A possible adverse reaction to carbamazepine. Am J Gastroenterol 1997;92:2126-7. [ Links ]
39. Faresjo A, Grodzinsky E, Johansson S, et al. Self-reported use of pharmaceuticals among patients with irritable bowel syndrome in primary care. J Manag Care Pharm 2008;14:870-7. [ Links ]
40. Nyhlin N, Bohr J, Eriksson S, et al. Systematic review: Microscopic colitis. Aliment Pharmacol Ther 2006;23:1525-34. [ Links ]
41. Vigren L, Sjoberg K, Benoni C, et al. Is smoking a risk factor for collagenous colitis? Scand J Gastroenterol 2011;46:1334-9. [ Links ]
42. Nyhlin N, Montgomery SM, Wickbom A, et al. Symptom burden in collagenous and lymphocytic colitis compared to a matched control group. Gut 2009;58(Supl. II):A309. [ Links ]
43. Yen EF, Pokhrel B, Du H, et al. Current and past cigarette smoking significantly increase risk for microscopic colitis. Inflamm Bowel Dis 2012;18:1835-41. [ Links ]
![]() Correspondence:
Correspondence:
Danila Guagnozzi.
Department of Gastroenterology.
Hospital General de Tomelloso.
Vereda de Socuéllamos, s/n.
13700 Tomelloso, Ciudad Real.
Spain
e-mail:
danila_g@libero.it
Received: 15-02-2015
Accepted: 05-04-2015