Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.7 Madrid jul. 2015
ORIGINAL PAPERS
Initial experience with fecal microbiota transplantation in Clostridium difficile infection - transplant protocol and preliminary results
Ana Ponte1, Rolando Pinho1, Margarida Mota2, Joana Silva1, Nuno Vieira2, Rosa Oliveira2, Teresa Pinto-Pais1, Carlos Fernandes1, Iolanda Ribeiro1, Jaime Rodrigues1, Paulo Lopes3, Tiago Teixeira2 and João Carvalho1
1Departments of Gastroenterology, 2Infection Control Group (Programa de Prevenção e Controlo de Infeção e Resistência aos Anti-microbianos) and 3Clinical Pathology. Centro Hospitalar Vila Nova de Gaia/Espinho. Portugal
ABSTRACT
Background and aims: Clostridium difficile infection (CDI) constitutes an important cause of antibiotic-associated diarrhea. Recurrence after first-line treatment with antibiotics is high and fecal microbiota transplantation (FMT) may be effective for refractory and recurrent CDI. This series aims to describe the efficacy of FMT in the treatment of refractory and recurrent CDI.
Methods: A prospectively recorded single-centre case series of patients with persistent or recurrent CDI treated with FMT between June 2014 and March 2015 was analyzed. Primary and secondary outcomes were defined as resolution of diarrhea without recurrence of CDI within 2 months after one or more FMT, respectively. A descriptive analysis was performed.
Results: 8 FMT were performed in 6 patients, 3 with refractory CDI and 3 with recurrent CDI. The median age of recipients was 71 years and 66.7% were women. One FMT was delivered through colonoscopy and the remaining 87.5% through esophagogastroduodenoscopy. One upper FMT was excluded due to recurrence of CDI after antibiotic exposure for a respiratory infection. The overall cure rate of FMT was total with lower route and 83.3% with upper route. Primary cure rate was achieved in 83.3% of patients and secondary cure rate was achieved in all patients. Median time to resolution of diarrhea after FMT was 1 day and no complications were reported during follow-up.
Conclusion: FMT appears to constitute a safe and effective approach in the management of refractory and recurrent CDI. Difference between primary and secondary cure rates may result of insufficient restoration of intestinal microbiota with a single FMT.
Key words: Clostridium difficile infection. Fecal microbiota transplantation. Gut microbiota.
Introduction
Since its first description in 1978, the incidence of Clostridium difficile infection (CDI) has steadily increased to epidemic proportions, mainly over the past 15 years, constituting the leading cause of antibiotic-associated diarrhea (1-6). The emergence of more virulent Clostridium difficile strains including North American pulsed-field gel electrophoresis type 1, restriction endonuclease analysis group BI, and PCR ribotype 027 (NAP1/BI/027) associated with host factors such as older age, inflammatory bowel disease, immunecompromised status, recent abdominal surgery and prolonged hospitalization have been implicated in a rise in morbidity and mortality of CDI (3,6-8). Moreover, proton-pump inhibitor (PPI) therapy, prolonged hospitalization, antibiotic treatment, renal failure and nasogastric tube feeding predispose to recurrent CDI (1). Although elderly and hospitalized patients receiving antibiotics are more commonly affected by CDI, it is increasingly acknowledged that young and healthy individuals with no previous exposure to antibiotics or hospitals can also develop CDI (3,5).
Currently, the first-line approach for primary CDI episodes is cessation, if possible, of the antibiotic related to CDI and a 10 to 14 day course of oral metronidazole or vancomycin, resulting in a clinical response in more than 80% of patients (1,5,8-10). Despite this initial response, recurrence ranges from 15 to 35%, and up to 45% will suffer a second recurrence and up to 65% will be affected by a third episode of CDI (2,5,11). Recurrent CDI is usually managed with an additional course of metronidazole, oral vancomycin, oral vancomycin in a pulsed and/or tapered regimen or with novel agents including fidaxomicin (1,2,5). The poor treatment outcomes for CDI prompted the investigation for alternative or adjunctive approaches to conventional antibiotics, such as probiotics, toxin-binding molecules, immunoglobulin and Clostridium difficile vaccination (2,10,12). Unfortunately, none of them has proven to be highly effective, safe and inexpensive to date (1,3). In contrast, fecal microbiota transplantation (FMT) appears to be a safe, highly effective and relatively inexpensive approach for refractory and recurrent CDI, which lead to its recent increased popularity despite its application in humans being described in the literature for about 50 years (1,3,4,11,12). Antibiotics disrupt the normal microbiota that suppresses the proliferation of Clostridium difficile in the colon, resulting in the production of toxins that cause damage to colonic epithelial cells leading to inflammation and symptoms (3,8). Moreover, due to the great resistance of Clostridium difficile spores to antibiotics, after cessation of the treatment, spores germinate into the vegetative forms, perpetuating CDI (3). FMT aims to restore the normal microbiota of the recipient, inducing a balance in the colonic flora and allowing host defense against CDI by preventing growth of toxigenic Clostridium difficile strains, resulting in reestablishment of the normal bowel function (6,8,13).
This series aims to describe the preliminary experience in our centre with FMT concerning efficacy in the treatment of refractory and recurrent CDI.
Material and methods
A prospectively recorded single-centre case series of the initial patients with CDI treated for persistent or recurrent CDI with FMT between June 2014 and March 2015 in Centro Hospitalar Vila Nova de Gaia/Espinho (Portugal) was analyzed. The protocol of FMT and its applicability in patients with refractory and recurrent CDI was approved by the Hospital Board. Written informed consent was provided by all patients or their legal representative before FMT after discussion of the expected benefits and potential adverse events. All patients were prospectively recorded in a data base and followed-up.
Definitions
CDI was defined as the presence of diarrhea, consisting of 3 or more unformed stools in 24 or fewer consecutive hours; a stool test result positive for the presence of toxigenic Clostridium difficile or its toxins or colonoscopic or histopathologic findings demonstrating pseudomembranous colitis (14). The same criteria were applied to the diagnosis of recurrent CDI (14). Recurrent or relapsing CDI was defined as at least three episodes of mild-to-moderate CDI and failure of a 6-8 week taper with vancomycin with or without an alternative antibiotic or at least two episodes of severe CDI resulting in hospitalization and associated with significant morbidity. Refractory CDI was defined as a moderate episode not responding to vancomycin for at least a week or a severe episode with no response to vancomycin after 48 hours (3).
Recipient
Patients were included if they were 18 years or older and fulfilled one of the primary indications for refractory or recurrent CDI as defined above.
Exclusion criteria due to increased risk of adverse events from treatment with FMT included patients on major immunosuppressive agents including high-dose corticosteroids, calcineurin inhibitors, mTOR inhibitors, lymphocyte depleting biologic agents, anti-tumor necrosis factor agents, and others; chemotherapeutic anti-neoplastic agents; patients with decompensated liver cirrhosis, advanced human immunodeficiency virus (HIV), recent bone marrow transplant, or other cause of severe immunodeficiency (3).
The information collected from each recipient included gender; age; use of chronic PPI, low-dose corticosteroids; enteral feeding through nasogastric tube; previous antibiotics; number of previous episodes of CDI before FMT; time from initial episode of CDI and FMT; white blood cells, creatinine level, pre-admission albumin level of the episode in which FMT was performed; prior treatments to CDI; route of delivery of FMT; cure rate of FMT; adverse events after FMT.
Donor
Donors were unrelated volunteers who underwent a screening based on medical history and laboratory testing (3,5). Exclusion criteria included known or recent exposure to HIV, hepatitis B or C; high-risk sexual behaviors; use of illicit drugs; tattoo or body piercing within 6 months; known current communicable disease; travel within the last 6 months to areas with endemic diarrheal illnesses; history of inflammatory bowel disease; history of irritable bowel syndrome, idiopathic chronic constipation or chronic diarrhea; history of gastrointestinal malignancy or known polyposis; antibiotics within the preceding 3 months; major immunosuppressive medications, including calcineurin inhibitors, high dose corticosteroids, biologic agents and chemotherapeutic agents (3,5). Moreover, morbid obesity, metabolic syndrome, atopy and chronic fatigue syndrome were additional exclusion criteria due to the risk of transmission by inoculation with intestinal microbiota of the donor (2). Due to a possible association with specific changes in the intestinal microbiota, donors with a history of major gastrointestinal surgery or systemic autoimmunity (multiple sclerosis, connective tissue disease) were also excluded (2). Donor blood testing included syphilis, HIV, hepatitis A, B and C and donor stool testing included Clostridium difficile toxin, bacterial culture for enteric pathogens, ova and parasites, Giardia antigen and cryptosporidium antigen (3,5).
FMT Protocol
Donor preparation
Donors were given lactulose the day before the procedure and were instructed to report any symptoms suggestive of infection which had appeared after their screening (3).
Stool preparation
After collection of the donor fecal material, stools were suspended in a non-bacteriostatic saline and mixed manually to homogenize the suspension, with a proportion of 50 g of feces in each 250 mL of diluent. A quantity of 25-50 mL and 250-500 mL was used for administration through upper endoscopy or colonoscopy, respectively. The mixture was subsequently filtered through gauze pads to eliminate particulate matter in order to avoid obstruction of the biopsy channel and was collected into 50 cc opaque syringes (2,3,12) (Fig. 1). Donor feces were collected within a maximum of 24 hours before the FMT, and after preparation of the donor stool, the fecal material was refrigerated in 2-8 oC until FMT.
Recipient preparation
Regardless of the instillation route, all patients remained on the antibiotics prescribed for CDI until the day before the FMT and received bowel preparation with 4L of polyethylene glycol the night before the procedure (3,5,12). A dose of 40 mg of pantoprazole was given intravenously to the recipient the evening before and the morning of the procedure if an upper gastrointestinal endoscopy was considered (3). When the FMT was instilled during a colonoscopy, 2 mg of oral loperamide was previously administered to promote retention of FMT (2,3).
Instillation route
Administration of FMT was performed through an upper route using upper gastrointestinal endoscopy and a lower route using colonoscopy. When administration of FMT was performed trough an upper route using upper gastrointestinal endoscopy, sedation was not applied as the procedure is simple and takes an identical time as a standard diagnostic upper gastrointestinal endoscopy. When FMT was delivered using colonoscopy, deep sedation was performed to provide comfort to the patient as the procedure is time consuming.
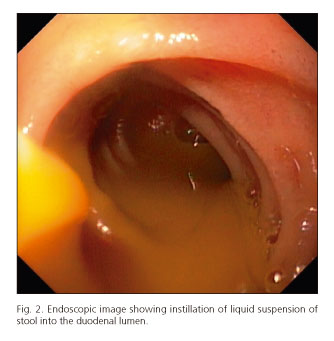
When the upper approach was performed, the endoscope was advanced with minimal air insufflation to the distal duodenum, where 50 mL of liquid suspension of stool was directly instilled through the biopsy channel of the endoscope into the duodenal lumen (Fig. 2), followed by a flush with 25 mL of water. The endoscope was subsequently withdrawn with air aspiration only in the stomach to avoid distension and minimize the risk of vomiting (15).
When the FMT was performed by colonoscopy, the colonoscope was inserted to the terminal ileum and each 50 cc syringe with 50 mL of liquid suspension of stool was directly instilled through the biopsy channel of the colonoscope into the terminal ileum, cecum, ascending colon, transverse colon, descending colon and sigmoid colon as the colonoscope was removed (16). The rectum was spared to avoid fecal urgency.
Outcomes
The primary outcome was defined as resolution of diarrhea without recurrence of CDI within 2 months after FMT and secondary outcome was defined as resolution of diarrhea without recurrence of CDI within 2 months after a further FMT. Recurrence was defined as relapse of symptoms of CDI after initial resolution of diarrhea within 2 months after FMT.
Statistical analysis
A descriptive analysis was performed using medians, ranges and percentages using the SPSS statistical package (version 20.0; IBM Corp., Armonk, New York, USA).
Results
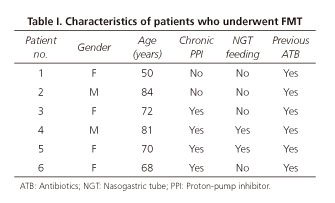
A total of 8 FMT were performed in 6 patients. According to table I, the majority of the recipients were women (66.7%) and the median age of the patients was 71 years, ranging from 50 to 84 years. All patients had a recent history of treatment with antibiotics, one patient was on low dose corticosteroid (prednisone 10 mg/day), 66.7% (4/6) reported chronic use of PPI and 33.3% (2/6) had a nasogastric tube for enteric nutrition. After diagnosis of CDI, analysis of NAP1/BI/027 was not performed in any patient. As summarized in table II, all patients received previous treatment with metronidazole, vancomycin and/or fidaxomicin, according to the severity and number of episodes of CDI. FMT was performed in 3 patients with a refractory primary episode, 2 of them with mild-to-moderate CDI and the third one with severe CDI. In the remaining 3 patients, the indication for FMT was recurrent CDI, in which 2 of them had at least two episodes of severe CDI and the other one had at least three episodes of mild-to-moderate CDI. Five patients were submitted to one FMT and one patient was submitted to three FMT, including one through a lower gastrointestinal route. Regarding the route of FMT, 87.5% (7/8) were delivered through upper gastrointestinal endoscopy and the remaining one through colonoscopy. Despite transient resolution of diarrhea, one case of upper FMT was excluded from the analysis because the patient experienced recurrence of CDI after an antibiotic course due to a respiratory infection administered after FMT. The overall cure rate of FMT was total (1/1) when a lower route with colonoscopy was used and was 83.3% (5/6) when upper gastrointestinal endoscopy was used. Primary cure rate was achieved in 83.3% (5/6) of patients and secondary cure rate was achieved in all patients. Median time of resolution of diarrhea after FMT was 1 day, ranging from 1 to 2 days. No adverse events were reported immediately after FMT and during the follow-up time.
Discussion
FMT consists of an infusion of a liquid suspension of stool from a healthy individual into the gastrointestinal tract of a receptor to cure a specific pathology (2,17,18). Therefore, FMT can be considered a form of organ transplantation although logistically simpler to perform than other organ transplants, as it does not require immunologic matching of donor and receptor and immunosuppression after the procedure (7,17).
Current literature on FMT reports overall cure rates that range from 81% to 100% (2). A recent systematic review concluded that 92% of patients had resolution of symptoms of CDI, 89% after a single treatment and 5% after retreatment due to failure or relapse (10). In our study, FMT achieved a primary cure rate of 83.3% and a secondary cure rate of 100% for refractory and recurrent CDI. The differences between primary and secondary cure rates may result of insufficient restoration of intestinal microbiota with only one FMT, which may be accomplished with furthers FMT (5). In fact, FMT induces a sustained microbial diversity in the recipient resembling that of the donor, characterized by an increased in Bacteroidetes species and Firmicutes and a decreased in the Proteobacteria species (2,13).
Several approaches for instillation of FMT have been described, including nasogastric or nasojejunal tube, upper gastrointestinal endoscopy, colonoscopy or retention enema (5,6,8,19). The optimal procedure for delivery of fecal material remains to be established (2,6). In our study, the overall cure rate of FMT was 83.3% when upper gastrointestinal endoscopy was used and was also achieved in the patient in whom a lower route with colonoscopy was used. Other studies report that the use of upper gastrointestinal endoscopy, nasogastric tube or nasoduodenal tube as instillation route of FMT resolved CDI in 76% to 79% of patients in contrast to 91% when FMT was delivered through colonoscopy (10,19). Despite studies suggest a slight superiority of efficacy of FMT administered through colonoscopy, a statistical significance was not achieved (6). A nonsignificant improved outcome is observed in patients who received higher amounts of FMT, which may possibly explain lower cure rates associated with the upper tract FMT where smaller stool volumes are provided to minimize the risk of vomiting and aspiration (1,2,10,18,19). Standard practices favor the use of 300 mL for colonic FMT and 60 mL to 75 mL for upper tract FMT and the dilution of 50 g of donor stools in 250 mL of diluent (2,18). Although colonoscopy has the advantage of delivering a large volume of FMT to the colon which is also examined allowing characterization of extent and severity of CDI and exclusion of other pathology, the procedure is time and resource consuming and carries other risks such perforation, when compared to upper routes (2,5,6,17). Therefore, nasogastric route or other upper instillations of FMT may be offered to mild-to-moderate recurrent CDI and colonoscopy may be reserved for severe cases of recurrent CDI (8). Nevertheless, in our protocol, regardless of the severity of CDI, upper gastrointestinal endoscopy-guided FMT was the preferred route due to its simplicity when compared to colonoscopy, which was reserved for patients in which upper endoscopy guided-TMF was not successful.
During the follow-up period after FMT of our study, no adverse events were reported including in the patient on low-dose corticosteroids. Transient gastrointestinal symptoms or altered bowel habits have been reported in some patients for several days after FMT, including nausea, abdominal pain, absence of bowel movement, bloating, flatulence, rectal discomfort (2,13,20,21). Furthermore, fever, paresthesia, gastroenteritis, transient sore throat and headache were also described (20,22). Although FMT appears to be safe with no adverse events or complications directly recognized, further studies are required to evaluate safety concerns of FMT, including risk of development of infectious or autoimmune disorders (2,5,18,19). Currently, risk of infection transmission and possible transmission of non-infectious gastrointestinal or systemic diseases of the donor are minimized through the application of a thorough clinical examination including a careful history, physical examination and laboratory analysis (2,7,19). Moreover, despite the fact that the use of FMT in immunosuppressed patients has been limited for safety concerns, recent studies suggest that FMT appears to be effective and safe in these patients (11,18).
Regardless increasing evidence supporting the safety and effectiveness of FMT, the unappealing nature of the procedure for patients and physicians may constitute a main factor that prevents its wide implementation and early use in the treatment of CDI (7,9). Nevertheless, a long-term follow up study concluded that 97% of patients who had undergone FMT for recurrent CDI would perform another FMT in the future if they had indication for it and 53% would choose FMT before antibiotics (2,5).
The absence of a standard approach and guidelines of FMT leads to important issues that should be addressed, which comprise use of large-volume bowel lavage to recipients before FMT, use of PPI in upper routes, appropriate time for cessation of antibiotics prescribed for CDI, appropriate type and amount of diluent used in the stool preparation and the relationship of the donor and the recipient.
In our study, all recipients received a large-volume bowel lavage before FMT with the purpose of reducing pathogenic organisms, improving colonization of donor fecal microbiota (2,3,13). Further studies are required to prove this concept (2). In our protocol, recipients who underwent upper endoscopy received PPI the evening before and the morning of the procedure to attenuate acid gastric secretion that could affect the donor fecal microbiota instilled (2). In opposition to the well-known association of chronic PPI use and CDI, the effect in outcome of the transient use of PPI in FMT needs to be determined (2). In our study, all patients remained on the antibiotics prescribed for CDI until the day before FMT. Other studies, anticipate the cessation of antibiotics 2 to 3 days before FMT (2). Currently, studies comparing both approaches are lacking (2).
Despite some studies suggesting a slightly higher cure rate when donors are related to their recipients, recent data advocate comparable cure rates of FMT between related and unrelated donors (2,6,7,18,19). In our protocol, unrelated donors were used to minimize the costs of screening, as one unrelated donor would be eligible for several recipients. Although current practices establish the use of fresh stool provided by the donor within 24 hours and preferably within 6 hours of FMT, recent studies suggest comparable cure rates in patients with recurrent CDI who underwent FMT with fresh stool and FMT with previously frozen stools (2,17,19). More studies are warranted to confirm the feasibility of FMT with frozen donor stools, which may open the possibility for the creation of fecal donor banks (2,17,19). The effectiveness of the diluents used to prepare donor feces is difficult to interpret because suspensions of FMT prepared with water, normal saline and milk resulted in cure rates of 98.5%, 86.2% and 88.6%, respectively and recurrence rates of 7.8%, 3% and 3.2%, respectively (2,10,18,19). Thus, water solution may result in higher resolution and recurrence rates in contrast to normal saline and milk solutions (2,10,18,19). Future studies are necessary to clarify this point, as the existing studies regarding this matter are heterogeneous (2).
The therapeutic benefit of FMT in CDI is promoting the investigation of the applicability of FMT to other disorders where gut microbiota dysfunction may play a role including inflammatory bowel disease, irritable bowel syndrome, metabolic syndrome, autoimmune and allergic diseases and neurodevelopmental disorders (2,4,5,7). Moreover, future directions for FMT may encompass the use of capsulized frozen FMT inocula that can be administered orally, which may obviate the need for invasive procedures for administration, decreasing costs and risks related to the procedure itself (23).
In conclusion, FMT appears to constitute a safe, simple and effective approach in the management of refractory and recurrent CDI. Further studies are warranted to clarify safety and procedural issues which were discussed above, in order to implement a standard protocol of the technique and to determine the role of FMT in the treatment algorithm of CDI.
References
1. Rubin TA, Gessert CE, Aas J, et al. Fecal microbiome transplantation for recurrent Clostridium difficile infection: report on a case series. Anaerobe 2013;19:22-6. DOI: 10.1016/j.anaerobe.2012.11.004. [ Links ]
2. Brandt LJ, Aroniadis OC. An overview of fecal microbiota transplantation: Techniques, indications, and outcomes. Gastrointest Endosc 2013;78:240-9. DOI: 10.1016/j.gie.2013.03.1329. [ Links ]
3. Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011;9:1044-9. DOI: 10.1016/j.cgh.2011.08.014. [ Links ]
4. Kelly CR, Kunde SS, Khoruts A. Guidance on preparing an investigation new drug application for fecal microbiota transplantation studies. Clin Gastroenterol Hepatol 2014;12:283-8. DOI: 10.1016/j.cgh.2013.09.060. [ Links ]
5. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012;107:1079-87. DOI: 10.1038/ajg.2012.60. [ Links ]
6. Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am J Gastroenterol 2013;108:500-8. DOI: 10.1038/ajg.2013.59. [ Links ]
7. Borody TJ, Khoruts A. Fecal microbiota transplantation and emergin applications. Nat Rev Gastroenterol Hepatol 2011;9:88-96. DOI: 10.1038/nrgastro.2011.244. [ Links ]
8. Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: A review and pooled analysis. Infection 2012;40:643-8. DOI: 10.1007/s15010-012-0307-9. [ Links ]
9. Zipursky JS, Sidorsky TI, Freedman CA, et al. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis 2012;55:1652-8. DOI: 10.1093/cid/cis809. [ Links ]
10. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011;53:994-1002. DOI: 10.1093/cid/cir632. [ Links ]
11. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014;109:1065-71. DOI: 10.1038/ajg.2014.133. [ Links ]
12. Di Bella S, Drapeau C, García-Almodóvar E, et al. Fecal microbiota transplantation: The state of the art. Infect Dis Rep 2013;5:e13. DOI: 10.4081/idr.2013.e13. [ Links ]
13. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407-15. DOI: 10.1056/NEJMoa1205037. [ Links ]
14. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55. DOI: 10.1086/651706. [ Links ]
15. Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: Ready for prime time? Cleve Clin J Med 2013;80:101-8. DOI: 10.3949/ccjm.80a.12110. [ Links ]
16. UNC Fecal Microbiota Transplant (FMT) Protocol for Refractory Clostridium Difficile Infection (CDI). Available at: http://www.med.unc.edu/gi/faculty-staff-website/patient-education/1FecalTransplantProtocols.pdf (Access in 10th March 2015). [ Links ]
17. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: A systematic review. J Clin Gastroenterol 2014;48:693-702. DOI: 10.1097/MCG.0000000000000046. [ Links ]
18. Brandt LJ. American Journal of Gastroenterology Lecture: Intestinal microbiota and the role of fecal microbiota transplantation (FMT) in treatment of C. difficile infection. Am J Gastroenterol 2013;108:177-85. DOI: 10.1038/ajg.2012.450. [ Links ]
19. Smits LP, Bouter KE, de Vos WM, et al. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013;145:946-53. DOI: 10.1053/j.gastro.2013.08.058. [ Links ]
20. Sha S, Liang J, Chen M, et al. Systematic review: Fecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther 2014;39:1003-32. DOI: 10.1111/apt.12699. [ Links ]
21. Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 2012;107:761-7. DOI: 10.1038/ajg.2011.482. [ Links ]
22. Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 2013;108:1367. DOI: 10.1038/ajg.2013.164. [ Links ]
23. Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014 ;312:1772-8. DOI: 10.1001/jama.2014.13875. [ Links ]
![]() Correspondence:
Correspondence:
Ana Ponte.
Departments of Gastroenterology.
Centro Hospitalar Vila Nova de Gaia/Espinho.
Portugal
e-mail:
ana.ilponte@gmail.com
Received: 14-03-2015
Accepted: 16-04-2015