My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.8 Madrid Aug. 2015
ORIGINAL PAPERS
Echoendoscopic characterization of the human colon
Fernando M. Castro-Poças1,2, Mário Dinis-Ribeiro3, Tarcísio P. Araújo1 and Isabel Pedroto1,2
1 Department of Ultrasound and Department of Gastroenterology. Santo António Hospital. Porto Hospital Center. Porto, Portugal.
2 Institute of Biomedical Sciences Abel Salazar. University of Porto. Portugal.
3 Center for Health Technology and Services Research. Faculty of Medicine. University of Porto. Portugal
ABSTRACT
Purpose: To characterize colon and rectum walls, pericolic and perirectal spaces, using endoscopic ultrasonography miniprobes.
Methods: Sixty individuals (50% males), aged 18-80, were included. Using 12 and 20 MHz endoscopic ultrasonography miniprobes, all different colon segments (ascending, transverse, descending, sigmoid) and rectum were evaluated according to the number and thickness of the different layers in intestinal wall, to the presence and (largest) diameter of vessels in the submucosa and of peri-intestinal nodes.
Results: The 20 MHz miniprobe identified a higher number of layers than the 12 MHz miniprobe, with medians of 7 and 5 respectively (p < 0.001). The rectal wall (p = 0.001), its muscularis propria (p < 0.001) and mucosa (p = 0.01) were significantly thicker than the different segments of the colon, which had no significant differences between them. Patients aged 41-60 presented thicker colonic wall and muscularis propria in descending (p = 0.001 and p = 0.004) and rectum (p=0.01 and p=0.01). Submucosal vessels were identified in 30% of individuals in descending and rectum, and in 12% in ascending. Adenopathies were observed in 9% of the colon segments and 5% in rectum.
Conclusions: A higher frequency enabled the identification of a higher number of layers. Rectal wall is thicker than the one from all the segments of the colon and there are no differences between these, namely in the ascending colon. Moreover, peri-intestinal adenopathies were rarely identified but present in asymptomatic individuals. All together, these results describe for the first time features which are relevant during staging and therapeutic management of colonic lesions.
Key words: Human colon. Miniprobes. Endoscopic ultrasonography. Intestinal wall.
Introduction
Although the use of endoscopic ultrasonography (EUS) in colonic pathology is well established for the assessment of subepithelial lesions and staging of rectal neoplasms, its use during staging of colon cancer (1,2) and management of patients with inflammatory bowel disease (3-5) is still a matter of debate and research. In fact, with the current techniques aiming at endoscopic resection of colonic lesions, such as mucosectomy and submucosa dissection, several authors defend the need to assess the lesion by EUS prior to these therapies (1,2,6-8) and compare the results obtained by different ultrasonographic frequencies (6,8). Moreover, colon wall thickness is sometimes a limiting factor in applying endoscopic resection techniques and may influence the management approach (9).
Nevertheless, the normal status of colonic and rectal wall and peri-intestinal space has not previously been described using EUS. Thus, we aimed to characterize colon and rectum walls, as well as pericolic and perirectal spaces, using EUS miniprobes with 12 and 20 MHz in a group of healthy subjects.
Material and methods
Study design and patients selection
This study was approved by our hospital's Health Ethics Committee. All subjects included in the study signed a written informed consent.
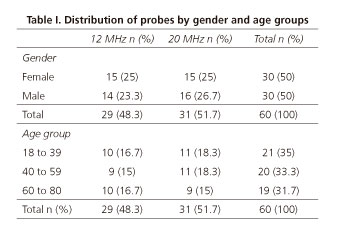
Sixty asymptomatic patients (50% being males, age varying between 18-80 years old) without current colorectal disease were included, according to three age groups: 18 to 40 (n = 21), 41 to 60 (n = 20) and 61 to 80 years old (n = 19); in each of these age groups, 50% were males.
Subjects between 41 and 80 years old were recruited in the Gastroenterology external service of our hospital among colon endoscopic polypectomy follow-up subjects and those submitted to colonoscopy for colon cancer screening; subjects between 18 and 40 years old were asymptomatic volunteers. All included subjects were asymptomatic and with no previous history of colorectal disease, except endoscopic polypectomy (Table I). Patients in which diverticular disease was identified during colonoscopy were excluded from the study.
Procedures
Bowel preparation was conducted using a standard intake of 4 liters of polyethylene glycol. EUS was performed using miniprobes equipped with 12 or 20 MHz transducers providing 360o radial images (Olympus UM-2R® and UM-3R®). All subjects underwent total colonoscopy and respective EUS under endovenous sedation with propofol, administered by an anesthesiologist.
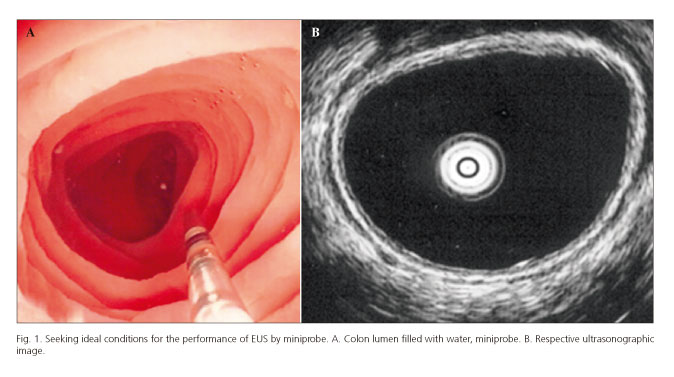
Ultrasonographic assessment was conducted in all segments -ascending (AC), transverse (TC), descending (DC), sigmoid (SC) colon and rectum (RE)- and data assessed separately. Colon segments and rectum were filled with water, in order to achieve an adequate acoustic window to view the entire circumference of intestinal wall (Fig. 1). We recognize that the presence of water in the intestinal lumen may interfere with wall thickness measurements, but this technique was applied equally for all segments and we poured only the minimum amount necessary to achieve proper acoustic window.
We performed four measurements, one for each of the four quadrants of the wall, and used the average as our final result. Half the patients were examined using 12 MHz probes and the other half using 20 MHz. For all patients, the peridigestive space was examined using a 12 MHz probe, due to larger tissue penetration capability at this frequency.
Variables
Parameters assessed: Number of layers identified in intestinal wall; thicknesses of mucosa, submucosa, muscularis propria and total wall; identification and determination of largest diameter of vessels in the submucosa and of peri-intestinal nodes.
Total wall was defined as the distance between the interface edge resulting from ultrasounds with epithelium and subserosa, serosa or adventitia.
Statistical analysis
Wilcoxon test was used in the assessment of the different measurements. The number of layers detected by both miniprobes (12 and 20 MHz) was compared by the median test, as the values were not individually paired.
Mann-Whitney and Kruskal-Wallis tests were used for comparisons between genders and age groups, respectively.
Friedman test was used to compare the number of layers in the different segments of the same subject (AC, TC, DC, SC and RE).
Statistical analysis admitted a type I error of 0.05.
Software used in the analysis was PASW® version 21.
Results
Number of layers in intestinal wall
When the results obtained with the 12 and 20 MHz probes were analyzed together, there were no significant differences among the segments assessed, whether in what concerns the total number of layers identified per segment (p = 0.30), whether in the identification of each of the layers: mucosa (p = 0.65); submucosa (p = 0.70); muscularis propria (p = 0.12); subserosa/serosa or adventitia (p = 0.96).
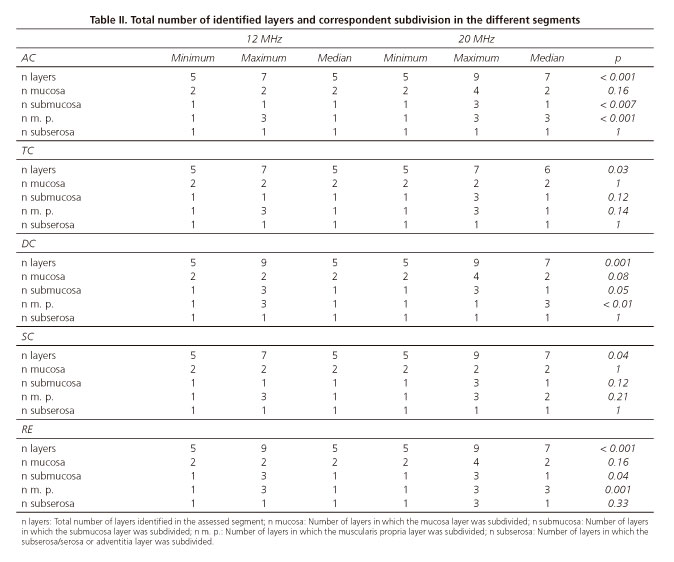
When we compared the assessments made by 12 and 20 MHz miniprobes, we observed statistically significant differences in all segments (Table II); 20 MHz miniprobe always identified a higher total number of layers. In what concerns the subdivision of the different layers, the behavior between segments is not uniform. In AC, DC and RE, submucosa and muscularis propria present a significantly higher number when observed by the 20 MHz miniprobe. In TC and SC, there are no significant differences in the subdivision of these layers when assessed at 12 or 20 MHz. At the level of mucosa and subserosa/serosa or adventitia, there were no significant differences between the 12 and 20 MHz miniprobe assessments, although the maximum number of layers in which the mucosa is subdivided is different: 4 by the 20 MHz miniprobe and 2 by 12 MHz in AC, DC and RE. In TC and SC, the value is equal: 2 layers.
Minimum and maximum numbers of identified layers by both frequencies were equal, respectively 5 and 9. In statistical terms, the number of layers more frequently found was 7 at 20 MHz.
The number of layers identified in the different segments was similar between genders and age groups, with no significant differences identified.
Intestinal wall thicknesses
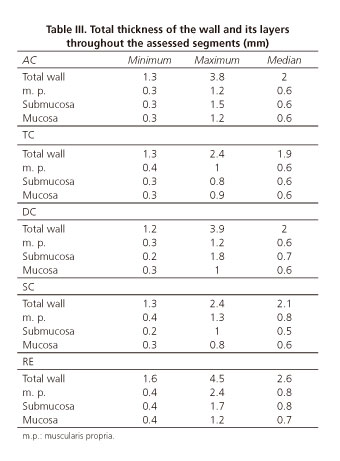
In table III we present the results of the total thickness of the intestinal wall and of its different layers. When we compared the different segments, we observed that statistically significant differences existed only between the thicknesses of the rectum and that of the different segments of the colon. Total thickness of the rectum wall (p = 0.001), muscularis propria (p < 0.001) and mucosa (p = 0.01) was larger than in the other segments of the colon. We did not observe any statistically significant difference in every calculation conducted regarding the wall thickness and its layers, depending on the frequency (12 or 20 MHz) used. We found a single statistically significant difference (p = 0.04) when comparing males and females: Muscularis propria of AC presented a larger thickness in woman than in man. In what concerns age, we observed that total wall and muscularis propria layer thicknesses were significantly higher in left colon (p-values of 0.01 and 0.004, respectively) and rectum (same p-value for both determinations, 0.01) in the age group ranging from 41 to 60 years old.
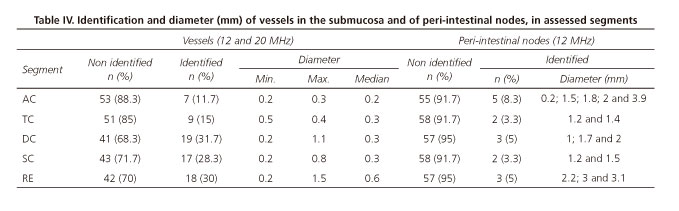
Identification of vessels in the submucosa
The characterization of vessels in the submucosa and its respective diameter distribution was conducted for the number of subjects for whom it was possible to identify those structures. We did not find any statistically significant difference regarding identification and diameter depending on the frequency used (12 or 20 MHz) and neither between gender and age groups and so the results are presented together (Table IV).
Identification of peri-intestinal nodes
In table IV, we also present the results of node identification in the different segments. Minimum and maximum diameters were of 0.2 and 3.9 mm, respectively, both cases in AC.
Discussion
Indications to conduct EUS in the colon are starting to emerge and correspondent findings may influence therapeutic decisions (1-3). Therefore, there is the need to create an echoendoscopic database concerning colon and rectum walls and surrounding spaces, for subsequent comparison with the results obtained in patients.
Number of layers identified in the intestinal wall
Some works refer the colon wall division into 5 layers by the 12 MHz (10) or 15 MHz frequencies (11), as well as the division into 9 layers by the 20 MHz frequencies (12). However, contrarily to ours, these have different objectives, not comparing different frequencies in the same segments and providing no other information besides the reference to the number of layers identified by a determined frequency.
The subdivision of the digestive wall in a different number of layers is not consensual; some authors identify a different number of layers with the same frequency (12,13), higher frequencies identifying a lower number of layers (12), and the same number of layers identified with much different frequencies (12,14).
These differences may be due to several reasons. The number of layers may change according to the organ assessed (13,15), to the degree of wall distension and/or compression or to its functional status (16). Another reason is the inter-observer variability, as we are facing an operator-dependent diagnosis method.
Another current problem is the correlation between the different layers observed in the digestive wall by EUS and its histological correspondence, namely when 9 or even 11 layers were identified (17,18).
Most of the authors (15-17) agree upon the correlation established between the different histological and echoendoscopic layers: The first two layers are related with the mucosa, the third with the submucosa, the fourth with muscularis propria, and the fifth layer with subserosa/serosa or adventitia. This depends on the location of the organ under analysis.
The subdivision of the intestinal wall in 9 layers, when identified by the 20 MHz probe, is interpreted by some authors (19) as follows: The first layer, hyperechogenic, corresponds to the interface between ultrasounds and epithelium; the second, hypoechogenic, corresponds to the epithelium; the third one, hyperechogenic, corresponds to the interface between the epithelium and the lamina propria and the lamina propria itself; the fourth, hypoechogenic, will be muscularis mucosa. The fifth layer, hyperechogenic, corresponds to the submucosa and its interfaces with muscularis mucosa and muscularis propria. The sixth, hypoechogenic, corresponds to the internal circular muscular layer. The seventh, hyperechogenic, is the interface between the two layers of the muscularis propria. The eighth, hypoechogenic, corresponds to the external longitudinal muscular layer. Finally, the ninth layer, hyperecogenic, corresponds to the interface between muscularis propria and peridigestive fat, and includes serosa or adventitia. We also found this wall division in 9 layers, dependant on the mucosa subdivision in the 4 mentioned layers, which was only possible at 20 MHz and in a limited number of assessments.
However, more frequently, our division into 9 layers was due to the submucosa layer subdivision into 3 layers (layers 1 and 2, mucosa; layers 3, 4 and 5, submucosa; 6, 7 and 8, muscularis propria; 9, subserosa/serosa).
The submucosa subdivision into 3 layers and its correlation with histology is one of the hardest findings to explain. This subdivision was also observed by another author, who does not give any interpretation for it (17). We believe that the very developed plexus of vessels existing in the submucosa (20) may be responsible for the submucosa ultrasonographic subdivision, which would justify the observation of 3 layers, from which the central one, hypoechogenic, would be the one related with vessels.
Colorectal wall division into 7 layers was our most frequent finding at 20 MHz. Correlation with histology would be as follows: The first layer, hyperecogenic, corresponds to the ultrasounds interface with the epithelium and superficial part of the mucosa; second layer, hypoechogenic, corresponds to the deep part of the mucosa; third layer, hyperecogenic, to the submucosa; the 3 following layers correspond to the muscularis propria, the fourth is the internal circular muscular, the fifth is the connective tissue beam, which separates it from the sixth, the external longitudinal muscular; finally, the seventh layer, that corresponds to the subserosa/serosa, or adventitia.
Median value at 20 MHz was 7 layers in all segments, with the exception of TC, in which it was 6. Median value at 12 MHz was 5 in all segments.
We did not observe any difference between genders or any other variation with age regarding the number of layers identified in the different segments.
Intestinal wall thickness and correspondent layers
There are still no standardized measures of the colon and rectum walls thickness determined by EUS. We found 4 works in the literature, all different in their methodology, in which, for the conduction of their studies, authors had the need to create a control group (21-24). Only two of them used EUS miniprobes and involved colon (23,24). The other two are limited to the rectum, one performed with rigid probe (22) and the other with an echoendoscope (21). None of them includes measurements for every wall layer and we couldn't even compare the thicknesses we measured in the colon because no reference was made to the measurement site in those studies. The thickness of the rectal wall was similar to our results (22).
When we compared the thicknesses of the different segments, we observed that there were statistically significant differences between the thicknesses of the rectum and all other colon segments, and no differences between the different colon segments. This latter finding contradicts the empirical idea that prevails among endoscopists that the AC walls have a lower thickness than the walls of the other colon segments (25).
Total thickness of the rectum wall (median 2.6 mm) was higher than the total thickness of the other segments of the colon (p = 0.001). There were no significant differences among these.
The same behavior was observed in muscularis propria thickness and it was significantly higher in the rectum than in the colon segments (p < 0.001). Submucosa was the only layer that did not present differences between any segments. At the level of the mucosa layer, there were significant differences (p = 0.01) between the thickness value in the rectum and the value obtained for all other colon segments.
Colorectal walls measurements were not influenced by the frequency used (12 or 20 MHz).
In what concerns the comparison between females and males, we observed a single significant difference (p = 0.04) in a particular segment and in a specific layer of intestinal wall: AC muscularis propria thickness is larger in females than in males. This difference had no reflection in the wall total thickness, which was the same for both genders. In what concerns the comparison between age groups, total wall and muscularis propria thicknesses in DC and RE were higher from 41 to 60 years old, and these differences were statistically significant. We do not know and did not find in the literature a response to the differences we observed between genders and in the 41 to 60 years old age group.
Identification of vessels in the submucosa
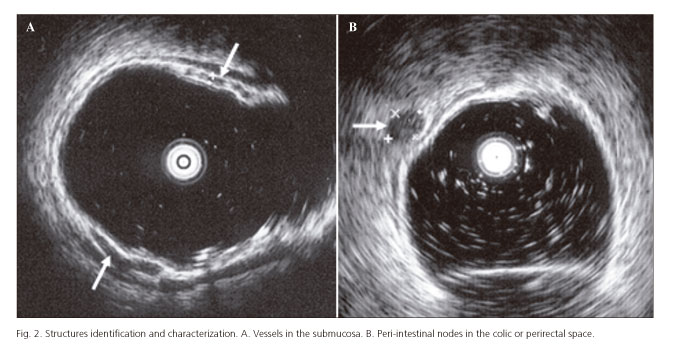
The identification of vessels in the submucosa (Fig. 2A) was not uniform in all segments, but lower values were registered in AC and TC than in DC, SC and RE. Therefore, in what concerns the identification of vessels in the submucosa, and only by the percentages in each segment, we could divide colon in two parts, AC and TC, with values between 11% and 15%, and all other segments with values around 30%. We did not observe a reason for these results. We didn't find any reference in the literature concerning identification and/or measurement of the vessels in the submucosa.
Identification of peri-intestinal nodes
Identification of nodes in colic and perirectal space (Fig. 2B) was very rare, with a maximum value of 8.3% (5 subjects) registered at AC level. There is a work that searched for nodes in asymptomatic controls (21). However, the study was limited to 20 subjects and to the perirectal space. Nodes were not found in any of the assessed controls.
Limitations
We recognize that the fact that all measurements were made by a single operator may be a limitation. However, the fact that the operator had an extensive experience of more than 15 years when the study was performed and that diverse sequential measurements were performed, may also allow us to assume that no significant bias occurred.
Conclusion
As expected, a higher frequency enabled the identification of an increased number of layers. Rectal wall is thicker than the one of all the segments of the colon and there are no differences between these, namely in the ascending colon.
Measurements of colorectal walls thickness were not influenced by the frequency used (12 or 20 MHz).
Identification of vessels in the submucosa is not uniform throughout the colon, and it is more frequent in left segments of intestine and rectum.
Moreover, the presence of peri-intestinal adenopathies was rarely identified in asymptomatic subjects.
All together, these results describe for the first time features which are relevant during staging and therapeutic management of colonic lesions.
References
1. Haji A, Ryan S, Bjarnason I, et al. Colonoscopic high frequency mini-probe ultrasound is more accurate than conventional computed tomography in the local staging of colonic cancer. Colorectal Dis 2012;14:953-9. DOI: 10.1111/j.1463-1318.2011.02871.x. [ Links ]
2. Gall TMH, Markar SR, Jackson D, Haji A, Faiz O. Mini-probe ultrasonography for the staging of colon cancer: A systematic review and meta-analysis. Colorectal Dis 2013;16:1-8. DOI: 10.1111/codi.12445. [ Links ]
3. Watanabe O, Ando T, EL-Omar EM, et al. Role of endoscopic ultrasonography in predicting the response to cyclosporin A in ulcerative colitis refractory to steroids. Dig Liver Dis 2009;41:735-9. DOI: 10.1016/j.dld.2009.03.014. [ Links ]
4. Rustemovic N, Cukovic-Cavka S, Brinar M, et al. A pilot study of transrectal endoscopic ultrasound elastography in inflammatory bowel disease. BMC Gastroenterol 2011;11:113-20. DOI: 10.1186/1471-230X-11-113. [ Links ]
5. Ellrichmann M, Wietzke-Braun P, Dhar S, et al. Endoscopic ultrasound of the colon for the differentiation of Crohn's disease and ulcerative colitis in comparison with healthy controls. Aliment Pharmacol Ther 2014;39:823-33. DOI: 10.1111/apt.12671. [ Links ]
6. Ichikawa T, Kudo M, Matsui S, et al. Endoscopic ultrasonography with three miniature probes of different frequency is an accurate diagnostic tool for endoscopic submucosal dissection. Hepatogastroenterology 2007;54:325-8. [ Links ]
7. Repici A, Pagano N. Endoscopic mucosal resection-endoscopic submucosal dissection: Do we really need endoscopic ultrasonography assistance? Minerva Med 2007;98:417-21. [ Links ]
8. Haji A, Ryan S, Bjarnason I, et al. High-frequency mini-probe ultrasound as a useful adjunct in the management of patients with malignant colorectal polyps. Colorectal Dis 2013;15:304-8. DOI: 10.1111/j.1463-1318.2012.03180.x. [ Links ]
9. Yoshida N, Yagi N, Naito Y, et al. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol 2010;16:1688-95. DOI: 10.3748/wjg.v16.i14.1688. [ Links ]
10. Akahoshi K, Yoshinaga S, Soejima A, et al. Transit endoscopic ultrasound of colorectal cancer using a 12 MHz catheter probe. Br J Radiol 2001;74:1017-22. DOI: 10.1259/bjr.74.887.741017. [ Links ]
11. Harada N, Hamada S, Kubo H. Preoperative evaluation of submucosal invasive colorectal cancer using a 15 MHz ultrasound miniprobe. Endoscopy 2001;33:237-40. DOI: 10.1055/s-2001-12798. [ Links ]
12. Saitoh Y, Obara T, Einami K, et al. Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc 1996;44:34-9. DOI: 10.1016/S0016-5107(96)70226-2. [ Links ]
13. Akahoshi K, Chijiiwa Y, Sasaki I, et al. Pre-operative TN staging of gastric cancer using a 15 MHz ultrasound miniprobe. Br J Radiol 1997;70:703-7. DOI: 10.1259/bjr.70.835.9245882. [ Links ]
14. Murata Y, Napoleon B, Odegaard S. High-frequency endoscopic ultrasonography in the evaluation of superficial esophageal cancer. Endoscopy 2003;35:429-36. DOI: 10.1055/s-2003-38774. [ Links ]
15. Palazzo L. Endosonographie digestive. Gastroentérologie 2002;10:1-15. [ Links ]
16. Calettti G, Bocus P, Togliani T, et al. The Gut Wall. In: Dam JV, Sivak MV, editors. Gastrointestinal endosonography. Philadelphia: Saunders; 1999. p. 102-3. [ Links ]
17. Ando M, Mochizuki F, Chonan A. Application of magnifying endoscopy and endoscopic ultrasonography to colorectal neoplastic lesions. Digest Endosc 2000;12:45-9. DOI: 10.1046/j.1443-1661.2000.0044a.x. [ Links ]
18. Cho E, Hasegawa K, Okabe Y, et al. Endoscopic ultrasonography for colorectal cancer. Digest Endosc 2001;13:19-21. DOI: 10.1046/j.1443-1661.2001.0130s1S19.x. [ Links ]
19. Wiersema M, Wiersema L. High resolution 25 megahertz ultrasonography of the gastrointestinal wall: Histologic correlates. Gastrointest Endosc 1993;39:499-504. DOI: 10.1016/S0016-5107(93)70159-5. [ Links ]
20. Standring S. Gray's Anatomy. 40th ed. Edinburgh: Churchill-Livingstone; 2008. p. 1134-5, 1161. [ Links ]
21. Gast P, Belaiche J. Rectal endosonography in inflammatory bowel disease: Differential diagnosis and prediction of remission. Endoscopy 1999;31:158-67. DOI: 10.1055/s-1999-13665. [ Links ]
22. Dagli U, Over H, Tezel A, et al. Transrectal ultrasound in the diagnosis and management of inflammatory bowel disease. Endoscopy 1999;31:152-7. DOI: 10.1055/s-1999-13664. [ Links ]
23. Tsuga K, Haruma K, Fujimura J, et al. Evaluation of the colorecal wall in normal subjects and patients with ulcerative colitis using an ultrasonic catheter probe. Gastrointest Endosc 1998;48:477-84. DOI: 10.1016/S0016-5107(98)70088-4. [ Links ]
24. Soweid A, Chak A, Katz J, et al. Catheter probe-assisted endoluminal US in inflammatory bowel disease. Gastrointest Endosc 1999;50:41-6. DOI: 10.1016/S0016-5107(99)70342-1. [ Links ]
25. Choo WK, Subhani J. Complication rates of colonic polypectomy in relation to polyp characteristics and techniques: A district hospital experience. J Interv Gastroenterol 2012; 2: 8-11. DOI: 10.4161/jig.20126. [ Links ]
![]() Correspondence:
Correspondence:
Fernando Castro-Poças.
Department of Ultrasound and
Department of Gastroenterology.
Santo António Hospital.
Porto Hospital Center.
Largo do Prof. Abel Salazar.
4099-001 Porto, Portugal
e-mail: castro.pocas@sapo.pt
Received: 06-02-2015
Accepted: 15-04-2015