My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.10 Madrid Oct. 2015
Impact of endoscopic monitoring in postoperative Crohn's disease patients already receiving pharmacological prevention of recurrence
Yago González-Lama, Isabel Blázquez, Cristina J. Suárez, Borja Oliva, Virginia Matallana, Marta Calvo, Isabel Vera and Luis Abreu
IBD Unit. Gastroenterology and Hepatology Department. Hospital Universitario Puerta de Hierro Majadahonda. Madrid, Spain
ABSTRACT
Background: Current guidelines address the initiation of treatment to prevent postoperative recurrence (PR) after ileo-cecal resection in Crohn's disease (CD), but appropriate management of postoperative CD patients who are already receiving treatment to prevent PR is yet to be defined. Usefulness of endoscopic monitoring in this scenario remains uncertain.
Aims: To evaluate the usefulness of endoscopy-based management of postoperative CD patients who are already under pharmacological prevention of PR.
Methods: Retrospective review of clinical outcome of all CD patients with ileo-cecal resection who underwent postoperative colonoscopy between 2004 and 2013 at our centre. Postoperative endoscopic findings were classified as no endoscopic recurrence (Rutgeerts i0-i1) or endoscopic recurrence (Rutgeerts i2-i4). Patients with endoscopic recurrence were classified as "endoscopy-based management (EBM)" if treatment step-up after endoscopy, or "non EBM (N-EBM)". Clinical recurrence was considered if re-operation, CD related hospitalization or treatment change. Time until clinical recurrence or the end of the follow up was considered.
Results: One hundred sixty six patients initially identified. One hundred twenty nine (77%) under pharmacological prevention of PR at the time of colonoscopy were analyzed: 34% were receiving aminosalicylates, 50% thiopurines, 11% anti-TNF, 5% combo. Colonoscopy showed endoscopic recurrence in 57% of patients; those with N-EBM were more likely to have clinical recurrence than patients with EBM along the follow up (p = 0.01).
Conclussions: Endoscopic monitoring could be useful in postoperative CD patients also in patients already receiving pharmacological treatment to prevent PR.
Key words: Crohn's disease. Recurrence. Endoscopy.
Introduction
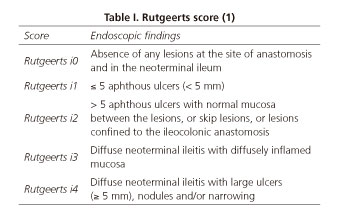
Crohn's disease (CD) is a chronic and disabling inflammatory bowel disease that needs surgical approach in many cases. Clinical recurrence of CD after surgery occurs in a significant proportion of patients, but endoscopic recurrence may affect to the vast majority. Postoperative prevention of recurrence remains an unresolved issue, although it is widely accepted that immunosuppressants should be recommended in those high-risk patients. Endoscopy seems to be a useful tool to predict postoperative behavior of the disease. Rutgeerts score (1) (Table I) is an endoscopic activity index that was designed to predict the clinical course of postoperative CD that is not under immunosuppressive therapy and, despite its lack of validation, is currently used to establish the need of postoperative pharmacological treatment with immunosuppressants for recurrence prevention.
On the other hand, being under pharmacological prophylaxis to prevent postoperative recurrence (PR) does not preclude a bad outcome in all cases (2-4). Moreover, postoperative CD patients in clinical remission may have endoscopic recurrence (5-7). Clinical relevance of mucosal healing in CD is widely accepted and is associated with better outcomes (8,9), and a recent prospective and randomized trial has shown that individualized immune suppression, adjusted for early endoscopic recurrence, leads to disease control in most cases (10); in spite of that, the need of endoscopic monitoring along the follow up in the postoperative scenario of patients who are already receiving treatment to prevent PR remains unclear. Endoscopy-based management of postoperative CD could implicate treatment changes to increase of immunosuppressive load according to endoscopic findings; whether or not Rutgeerts score is a useful tool also in this specific clinical scenario is still uncertain (8,9).
We designed a retrospective study aimed to evaluate the usefulness of endoscopy-based management in the real life clinical setting of postoperative CD patients who were already receiving pharmacological treatment to prevent PR.
Methods
A retrospective cohort study was designed including all of our CD patients with previous ileo-cecal resection who had undergone a postoperative colonoscopy at our center between 2004 and 2013. Data regarding each patient's demographics, characteristics of the disease, surgery, postoperative endoscopy and postoperative treatments were collected. We also reviewed the clinical outcome along the follow up of all CD patients included.
Postoperative endoscopy findings were classified as no endoscopic recurrence (Rutgeerts i0 or i1) or endoscopic recurrence (Rutgeerts i2, i3 or i4). Among those patients with endoscopic recurrence, we considered as "endoscopy-based management (EBM)" if the treatment was escalated, and "non endoscopy-based management (N-EBM)" in the rest of the cases. Any increase in the immunosuppressive load was considered as treatment escalation: from 5ASA to thiopurines or biologics; from thiopurines to biologics; if the patient was already receiving biologics, EBM was considered if dose intensification or switch to another biologic.
To assess the efficacy of those different strategies, we considered the time until clinical recurrence (re-operation, CD related hospitalization or CD treatment change) or the end of the follow up.
Statistical analysis
For quantitative variables, mean and standard deviation were calculated. For categorical variables, percentages and corresponding 95% intervals (95% CI) were provided. A p value < 0.05 was considered statistically significant. Categorical variables were compared with the χ2 test, and quantitative variables with the Student t test. Probability of remaining free of clinical recurrence was analysed using Kaplan-Maier survival curves, and they were compared using the Log-rank test, considering a p value < 0.05 as statistically significant.
Results
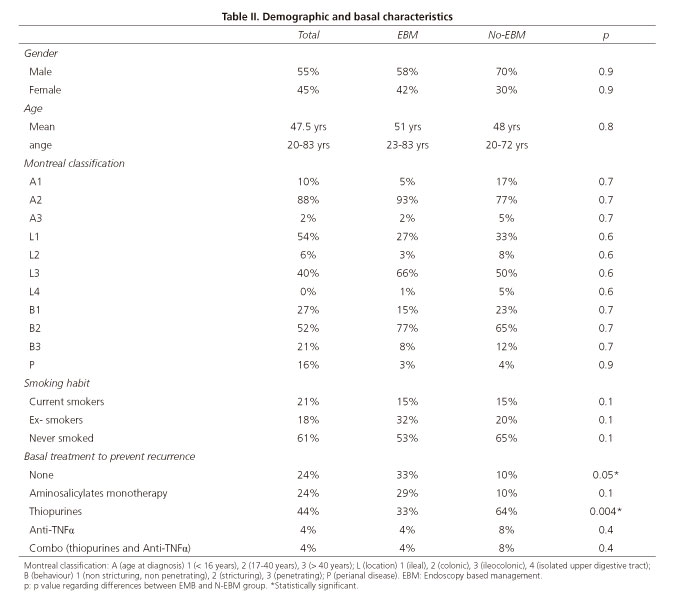
One hundred sixty six patients were initially evaluated. Most of these patients (129; 77%) were under pharmacological treatment to prevent PR at the time of the colonoscopy and were finally considered for the analysis; demographic and basal characteristics of patients considered for the analysis are summarized in table II. No statistically significant differences in demographics were found between EBM and N-EBM patients, although N-EBM patients had higher immunosuppressive load than those patients who received an EBM approach.
Time lapse between surgery and the colonoscopy that was considered for the analysis ranged between 2.5 and 420 months; mean time lapse was 112 months. There were no statistically significant differences in time lapse between surgery and colonoscopy among all different groups, including patients with an EBM and N-EBM. Postoperative colonoscopy showed no endoscopic recurrence (Rutgeerts i0 or i1) in 43% and endoscopic recurrence (Rutgeerts i2, i3 or i4) in 57%.
EBM approach was followed by 75% of our patients with endoscopic recurrence; time to clinical recurrence (as previously defined) in those patients according to their endoscopic findings is expressed in figure 1: the proportion of patients who remained free of clinical recurrence was 70%, 62%, 60%, 58% and 58% at the 1st, 2nd, 3rd, 5th and 9th year, respectively, while the proportion of patients with endoscopic recurrence who did not follow an EBM and remained free of clinical recurrence was 64%, 43%, 41%, 32%, 26% and 0% at the 1st, 2nd, 3rd, 5th and 9th year, respectively. There were statistically significant differences in the clinical behavior of those patients with endoscopic recurrence regarding the therapeutic approach: Patients with N-EBM were more likely to have clinical recurrence along the follow-up than those with endoscopic recurrence and EBM (p = 0.01).
Discussion
Intestinal resection is almost unavoidable in the natural history of CD, and as much as 80% of the patients would need surgery at any point of their follow-up. Since surgery is not curative, clinical PR occurs in a significant proportion of the patients who have undergone any kind of surgical resection (2); moreover, endoscopic recurrence of the disease is even much more frequent and may affect a majority of the patients in the first year after surgery and almost all of the patients after three years (5,6). Even though prophylaxis of PR in postoperative CD patients remains an unresolved issue in many aspects, it is widely accepted that patients with bad prognosis criteria should receive immunomodulators, and the immunosuppressive load should be selected according to the risk of clinical recurrence (2,11). In spite of all of that, patients are still not free from suffering a bad outcome (3,12).
Mucosal healing is an emerging concept in CD management. Even though healing of CD related mucosal lesions is associated to a better outcome in many ways, it has been hardly moved to clinical practice and it still remains unclear how much therapeutic effort should be done aiming this therapeutic target in each different clinical situations (9). Postoperative scenario is one of the most critical situations in which patients at risk of having a poorer outcome, or even a second resection, should be identified and probably be more aggressively treated (12,13).
Current guidelines are focused on initiation of PR prophylaxis, but further clinical management of patients who are already receiving pharmacological prophylaxis for PR needs to be addressed. It is still not clear if the presence of mucosal lesions can help clinicians to identify the group of patients that are at higher risk despite of an appropriate prophylaxis of PR, and if endoscopic findings could justify treatment step-up. Whether or not an endoscopy-based management should be advisable along the follow up of patients who have undergone surgical resection and are already receiving pharmacological prophylaxis to prevent PR, still needs to be clarified and is not supported by current guidelines (2,8,12).
A previous retrospective study failed to demonstrate the impact of an early postoperative colonoscopy (within the first year after surgery), but endoscopic findings were not standardized and immunosuppressive therapy was uncommon despite of treatment step-up (14). The group at Saint-Louis Hospital (France) retrospectively reviewed 132 postoperative CD patients and showed how a tailored treatment according to an early postoperative colonoscopy had a clear impact on the probabilities of having clinical recurrence at the 3rd and 5th year after surgery (15). Finally, the Postoperative Crohn's Endoscopic Recurrence (POCER) Study prospectively showed that adjusted immunosuppressive load for early recurrence based on a postoperative colonoscopy performed at the 6th month was superior to routine drug therapy alone in preventing PR in a 18 months period (10).
Our experience, that was not limited to early postoperative colonoscopies, showed that patients with endoscopic recurrence along the follow up did better if managed according to endoscopic findings: Half of the patients with endoscopic recurrence whose treatment was intensified remained free of clinical recurrence after 5 years of follow up, while half of those patients with endoscopic recurrence who did not modify their treatment presented clinical recurrence in less than 2 years of follow up. Our study, though retrospective, confirmed the findings of the POCER trial and suggested that treatment should be individually optimized according to endoscopic monitoring along the follow up. Interestingly, patients of the N-EBM group had higher immunosuppressive load than those who finally received an EBM approach; probably being under a high immunosuppressive load may have negatively influenced clinicians and precluded an EBM in some cases.
It has been suggested that mere clinical management of CD should be replaced for tight monitoring of more precise and objectively measurable parameters, since clinical symptoms hardly correlate with mucosal inflammation (16-18). Mucosal healing has been shown to be associated to a better outcome in CD patients (19-22); given that it is not always achievable, some evidence shows that at least endoscopic response may be sufficient to avoid a bad clinical outcome (23). Data in postoperative CD is scarce, but clinical features seem not to be a reliable marker of mucosal activity of the disease (7). Our results confirmed in real life clinical setting those results obtained by the POCER trial and supported the clinical impact of tight monitoring of mucosal lesions in the management of postoperative CD along the follow up, since early remission does not seem to preclude the need for ongoing monitoring (10).
In spite of all of that, some issues are still pending, such as the periodicity or the method to monitor the disease. Rutgeerts score is a widely known easy-to-use endoscopic activity index for postoperative CD that was designed to address the risk of clinical recurrence in patients under no treatment to prevent PR (8,24). The POCER study and our experience showed that Rutgeerts score was also useful to identify patients at risk of clinical recurrence despite of being under prophylaxis of PR, and treatment step-up was proposed for those patients who had Rutgeerts score ≥ i2 (8,10,25). The clinical relevance of i2 Rutgeerts was unclear and initially associated to intermediate risk (24); both POCER trial and our experience suggested that those slight mucosal changes should be taken into account in clinical practice.
Our study has a number of limitations related to the retrospective nature of the design. Indication of colonoscopies was clinically based and they were not performed under scheduled basis; for that reason, there was a wide range in the time lapse between surgery and the colonoscopy that was considered for the analysis and therefore impact of early endoscopic monitoring could not be specifically addressed.
In conclusion, endoscopic monitoring could be useful along the follow-up of postoperative CD despite being under pharmacological prophylaxis of PR and beyond the early postoperative period, since it may help to identify those patients who could take advantage of treatment step up. Nevertheless, further research is needed to firmly advocate for an endoscopic based management of postoperative CD patients under standardized basis.
References
1. Rutgeerts P, Geboes K, Vantrappen G, et al. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 1984;25:665-72. DOI: 10.1136/gut.25.6.665. [ Links ]
2. Van Assche G, Dignass A, Reinisch W, et al. The Second European Evidence-Based Consensus on the Diagnosis and Management of Crohn's Disease: Special Situations. J Crohns Colitis 2010;4:63-101. DOI: 10.1016/j.crohns.2009.09.009. [ Links ]
3. Swoger JM, Regueiro M. Evaluation for postoperative recurrence of Crohn disease. Gastroenterol Clin North Am 2012;41:303-14. DOI: 10.1016/j.gtc.2012.01.011. [ Links ]
4. Moss AC. Prevention of postoperative recurrence of Crohn's disease: What does the evidence support? Inflamm Bowel Dis 2013;19:856-9. [ Links ]
5. Buisson A, Chevaux JB, Allen PB, et al. Review article: The natural history of postoperative Crohn's disease recurrence. Aliment Pharmacol Ther 2012;35:625-33. DOI: 10.1111/j.1365-2036.2012.05002.x. [ Links ]
6. Orlando A, Mocciaro F, Renna S, et al. Early post-operative endoscopic recurrence in Crohn's disease patients: Data from an Italian Group for the Study of Inflammatory Bowel Disease (Ig-Ibd) Study on a large prospective multicenter cohort. J Crohns Colitis 2014;8:1217-21. DOI: 10.1016/j.crohns.2014.02.010. [ Links ]
7. Regueiro M, Kip KE, Schraut W, et al. Crohn's disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis 2011;17:118-26. DOI: 10.1002/ibd.21355. [ Links ]
8. Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014;24:367-78. DOI: 10.1016/j.giec.2014.03.005. [ Links ]
9. De Cruz P, Kamm MA, Prideaux L, et al. Mucosal healing in Crohn's disease: A systematic review. Inflamm Bowel Dis 2013;19:429-44. DOI: 10.1002/ibd.22977. [ Links ]
10. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn's disease management after intestinal resection: A randomised trial. Lancet 2015;385:1406-17. DOI: 10.1016/S0140-6736(14)61908-5. [ Links ]
11. Vaughn BP, Moss AC. Prevention of post-operative recurrence of Crohn's disease. World J Gastroenterol 2014;20:1147-54. DOI: 10.3748/wjg.v20.i5.1147. [ Links ]
12. van Lent AU, D'Haens GR. Management of postoperative recurrence of Crohn's disease. Dig Dis 2013;31:222-8. DOI: 10.1159/000353374. [ Links ]
13. Riss S, Schuster I, Papay P, et al. Repeat Intestinal resections increase the risk of recurrence of Crohn's disease. Dis Colon Rectum 2013;56:881-7. DOI: 10.1097/DCR.0b013e31828cb80c. [ Links ]
14. De Cruz P, Bernardi MP, Kamm MA, et al. Postoperative recurrence of Crohn's disease: Impact of endoscopic monitoring and treatment step-up. Colorectal Dis 2013;15:187-97. DOI: 10.1111/j.1463-1318.2012.03168.x. [ Links ]
15. Baudry C, Pariente B, Lourenco N, et al. Tailored treatment according to early post-surgery colonoscopy reduces clinical recurrence in Crohn's disease: A retrospective study. Dig Liver Dis 2014;46:887-92. DOI: 10.1016/j.dld.2014.07.005. [ Links ]
16. Papay P, Ignjatovic A, Karmiris K, et al. Optimising monitoring in the management of Crohn's disease: A physician's perspective. J Crohns Colitis 2013;7:653-69. DOI: 10.1016/j.crohns.2013.02.005. [ Links ]
17. Antunes O, Filippi J, Hebuterne X, et al. Treatment algorithms in Crohn's -up, down or something else? Best Pract Res Clin Gastroenterol 2014;28:473-83. [ Links ]
18. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the Sonic Trial. Gut 2014;63:88-95. DOI: 10.1136/gutjnl-2013-304984. [ Links ]
19. Baert F, Moortgat L, Van Assche G, et al. Mucosal Healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology 2010;138:463-8; quiz e10-1. DOI: 10.1053/j.gastro.2009.09.056. [ Links ]
20. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis 2009;15:1295-301. DOI: 10.1002/ibd.20927. [ Links ]
21. Osterman MT. Mucosal healing in inflammatory bowel disease. J Clin Gastroenterol 2013;47:212-21. DOI: 10.1097/MCG.0b013e3182732ff5. [ Links ]
22. Orlando A, Guglielmi FW, Cottone M, et al. Clinical implications of mucosal healing in the management of patients with inflammatory bowel disease. Dig Liver Dis 2013;45:986-91. doi: 10.1016/j.dld.2013.07.005. DOI: 10.1016/j.dld.2013.07.005. [ Links ]
23. Ferrante M, Colombel JF, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn's disease based on a post hoc analysis of data from Sonic. Gastroenterology 2013;145:978-986.e5. doi: 10.1053/j.gastro.2013.08.010. DOI: 10.1053/j.gastro.2013.08.010. [ Links ]
24. Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956-63. [ Links ]
25. Domenech E, Manosa M, Bernal I, et al. Impact of azathioprine on the prevention of postoperative Crohn's disease recurrence: Results of a prospective, observational, long-term follow-up study. Inflamm Bowel Dis 2008;14:508-13. DOI: 10.1002/ibd.20359. [ Links ]
![]() Correspondence:
Correspondence:
Luis Abreu.
IBD Unit. Gastroenterology and Hepatology Department.
Hospital Universitario Puerta de Hierro Majadahonda.
C/ Manuel de Falla, 1.
28222 Majadahonda, Madrid. Spain
e-mail: luisabreugarcia@gmail.com
Received: 06-05-2015
Accepted: 01-07-2015